Tuesday Poster Session
Category: IBD
P3536 - Serological Responses to SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Disease Are Impaired for Those on Anti-TNF and Immunomodulator Combination Therapy
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Kenneth Ernest-Suarez, MD
University of Calgary
Calgary, AB, Canada
Presenting Author(s)
Kenneth Ernest-Suarez, MD1, Catherine Rowan, MD1, Joshua Quan, MSc1, Fiona Yeaman, MBBS1, Christopher Ma, MD2, Remo Panaccione, MD1, Lindsay Hracs, PhD1, Nastaran Sharifi, MD1, Michelle Herauf, MSc1, Ante Markovinović, BA1, Stephanie Coward, PhD1, Joseph W.. Windsor, PhD1, Léa Caplan, BHSc1, Richard J. M.. Ingram, MD, PhD1, Cynthia Seow, MD1, Kerri Novak, MD1, Cathy Lu, MD, MSc1, Gilaad G. Kaplan, MD, MPH1
1University of Calgary, Calgary, AB, Canada; 2Alimentiv Inc., University of Calgary, London, ON, Canada
Introduction: Anti-tumor necrosis factor (TNF) reduces the serological responses (SR) to SARS-CoV-2 vaccination and impairs SR durability within individuals with IBD.1-3 Additional doses of SARS-CoV-2 vaccination effectively increase antibody titers in patients treated with anti-TNF agents, responses are still lower compared to healthy controls.4 5 Data comparing serological responses between anti-TNF monotherapy and combination therapy are lacking. This study aims to compare serological responses from SARS-CoV-2 vaccination between IBD patients treated with anti-TNF monotherapy or combined with an immunomodulator.
Methods: Adults with IBD, at least two doses of a SARS-CoV-2 vaccine, with anti-TNF monotherapy (anti-TNF alone) or combination therapy (anti-TNF in addition to methotrexate or azathioprine) were recruited through the STOP COVID-19 in IBD cohort.5 Serum samples were drawn for assessment of IgG antibodies to the spike protein of SARS-CoV-2 (anti-S) using the Abbott Architect SARS-CoV-2 IgG II Quant assay following 1–8 weeks after 1st dose vaccination, and both 1–8 weeks and 8+ weeks after 2nd, 3rd, and 4th dose vaccination. Demographic and medication status information was collected through chart review. SR was defined as IgG levels of ≥50 AU/mL; positive SR rates were compared between medication groups using two-sample proportion tests. Anti-S concentrations were reported as geometric mean titres (GMT) with 95% confidence intervals and were compared between medication groups using Mann Whitney-U tests.
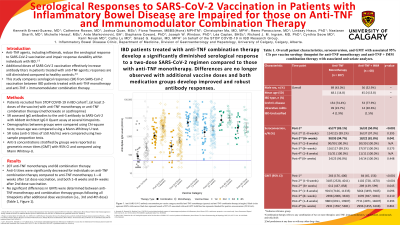
Results: 207 patients on anti-TNF monotherapy and 68 on combination therapy were recruited. Anti-S titres were significantly decreased for individuals on combination therapy compared to anti-TNF monotherapy 1–8 weeks after 1st dose vaccination, and both 1–8 weeks and 8+ weeks after 2nd dose vaccination. No significant differences in GMTs were determined between anti-TNF monotherapy and combination therapy groups following all timepoints after additional dose vaccination (Table 1, Figure 1).
Discussion: IBD patients treated with combination therapy develop a significantly reduced serological response to a two-dose SARS-CoV-2 regimen compared to those with anti-TNF monotherapy. These differences are no longer observed following additional vaccine doses and both medication groups develop robust antibody responses. These findings highlight the importance of additional doses for adequate serological protection within the IBD population and especially for those on anti-TNF combination therapy.

Disclosures:
Kenneth Ernest-Suarez, MD1, Catherine Rowan, MD1, Joshua Quan, MSc1, Fiona Yeaman, MBBS1, Christopher Ma, MD2, Remo Panaccione, MD1, Lindsay Hracs, PhD1, Nastaran Sharifi, MD1, Michelle Herauf, MSc1, Ante Markovinović, BA1, Stephanie Coward, PhD1, Joseph W.. Windsor, PhD1, Léa Caplan, BHSc1, Richard J. M.. Ingram, MD, PhD1, Cynthia Seow, MD1, Kerri Novak, MD1, Cathy Lu, MD, MSc1, Gilaad G. Kaplan, MD, MPH1. P3536 - Serological Responses to SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Disease Are Impaired for Those on Anti-TNF and Immunomodulator Combination Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Calgary, Calgary, AB, Canada; 2Alimentiv Inc., University of Calgary, London, ON, Canada
Introduction: Anti-tumor necrosis factor (TNF) reduces the serological responses (SR) to SARS-CoV-2 vaccination and impairs SR durability within individuals with IBD.1-3 Additional doses of SARS-CoV-2 vaccination effectively increase antibody titers in patients treated with anti-TNF agents, responses are still lower compared to healthy controls.4 5 Data comparing serological responses between anti-TNF monotherapy and combination therapy are lacking. This study aims to compare serological responses from SARS-CoV-2 vaccination between IBD patients treated with anti-TNF monotherapy or combined with an immunomodulator.
Methods: Adults with IBD, at least two doses of a SARS-CoV-2 vaccine, with anti-TNF monotherapy (anti-TNF alone) or combination therapy (anti-TNF in addition to methotrexate or azathioprine) were recruited through the STOP COVID-19 in IBD cohort.5 Serum samples were drawn for assessment of IgG antibodies to the spike protein of SARS-CoV-2 (anti-S) using the Abbott Architect SARS-CoV-2 IgG II Quant assay following 1–8 weeks after 1st dose vaccination, and both 1–8 weeks and 8+ weeks after 2nd, 3rd, and 4th dose vaccination. Demographic and medication status information was collected through chart review. SR was defined as IgG levels of ≥50 AU/mL; positive SR rates were compared between medication groups using two-sample proportion tests. Anti-S concentrations were reported as geometric mean titres (GMT) with 95% confidence intervals and were compared between medication groups using Mann Whitney-U tests.
Results: 207 patients on anti-TNF monotherapy and 68 on combination therapy were recruited. Anti-S titres were significantly decreased for individuals on combination therapy compared to anti-TNF monotherapy 1–8 weeks after 1st dose vaccination, and both 1–8 weeks and 8+ weeks after 2nd dose vaccination. No significant differences in GMTs were determined between anti-TNF monotherapy and combination therapy groups following all timepoints after additional dose vaccination (Table 1, Figure 1).
Discussion: IBD patients treated with combination therapy develop a significantly reduced serological response to a two-dose SARS-CoV-2 regimen compared to those with anti-TNF monotherapy. These differences are no longer observed following additional vaccine doses and both medication groups develop robust antibody responses. These findings highlight the importance of additional doses for adequate serological protection within the IBD population and especially for those on anti-TNF combination therapy.

Figure: Figure 1. Anti-SARS-CoV-2 antibody concentration per vaccine category stratified anti-TNF monotherapy (squares) and anti-TNF combination therapy (triangles). Black circles represent GMTs while narrow black bars represent bounds of 95% CI associated with each GMT. Solid blue line for positive seroconversion (50 AU/mL)
Disclosures:
Kenneth Ernest-Suarez: AstraZeneca – Advisor or Review Panel Member, Consultant. Ferring – Advisor or Review Panel Member, Consultant. Janssen – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Sandoz – Advisor or Review Panel Member, Consultant.
Catherine Rowan indicated no relevant financial relationships.
Joshua Quan indicated no relevant financial relationships.
Fiona Yeaman indicated no relevant financial relationships.
Christopher Ma: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. AVIR Pharma Inc – Consultant, Speakers Bureau. BioJAMP – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Ferring – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. McKesson – Consultant. Mylan – Consultant. Pendopharm – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Biosciences Inc. – Consultant. Roche – Consultant. Sanofi – Consultant. Springer Publishing – Royalties. Takeda – Consultant, Speakers Bureau.
Remo Panaccione: Abbivax – Consultant. Abbott – Consultant. AbbVie – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena – Consultant. AstraZeneca – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion – Consultant. Cosmos Technology – Consultant. Eisai – Consultant. Elan – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. JAMP Bio – Consultant. Janssen – Consultant. Merck – Consultant. Mylan – Consultant. Novartis – Consultant. Oppilan Pharma – Consultant. Organon – Consultant. Pandion Pharma – Consultant. Pendopharm – Consultant. Pfizer Inc – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist Therapeutics – Consultant. Roche – Consultant. Sandoz – Consultant. Satisfai Health – Consultant. Shire – Consultant. Sublimity Therapeutics – Consultant. Takeda Pharmaceuticals – Consultant. Theravance Biopharma – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Consultant. Viatris – Consultant.
Lindsay Hracs indicated no relevant financial relationships.
Nastaran Sharifi indicated no relevant financial relationships.
Michelle Herauf indicated no relevant financial relationships.
Ante Markovinović indicated no relevant financial relationships.
Stephanie Coward indicated no relevant financial relationships.
Joseph Windsor indicated no relevant financial relationships.
Léa Caplan indicated no relevant financial relationships.
Richard Ingram indicated no relevant financial relationships.
Cynthia Seow: Abbvie – Advisor or Review Panel Member, Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Consultant. Fresenius Kabi – Advisor or Review Panel Member, Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Kerri Novak: Abbvie – Advisor or Review Panel Member, Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Janssen – Advisor or Review Panel Member, Consultant. McKesson – Advisory Committee/Board Member. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support. Takeda – Advisor or Review Panel Member, Consultant.
Cathy Lu: Abbvie – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member.
Gilaad Kaplan: AbbVie (received honoraria) – Consultant, Speaking. Amgen (received honoraria) – Consultant, Speaking. Ferring – Grants for research support. Janssen (received honoraria) – Consultant, Speaking. Pendophram (received honoraria) – Consultant, Speaking. Pfizer (received honoraria) – Consultant, Speaking. Sandoz (received honoraria) – Consultant, Speaking. Takeda (received honoraria) – Consultant, Speaking. TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 Sept. – Intellectual Property/Patents.
Kenneth Ernest-Suarez, MD1, Catherine Rowan, MD1, Joshua Quan, MSc1, Fiona Yeaman, MBBS1, Christopher Ma, MD2, Remo Panaccione, MD1, Lindsay Hracs, PhD1, Nastaran Sharifi, MD1, Michelle Herauf, MSc1, Ante Markovinović, BA1, Stephanie Coward, PhD1, Joseph W.. Windsor, PhD1, Léa Caplan, BHSc1, Richard J. M.. Ingram, MD, PhD1, Cynthia Seow, MD1, Kerri Novak, MD1, Cathy Lu, MD, MSc1, Gilaad G. Kaplan, MD, MPH1. P3536 - Serological Responses to SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Disease Are Impaired for Those on Anti-TNF and Immunomodulator Combination Therapy, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

