Tuesday Poster Session
Category: IBD
P3538 - Associations Between Body Mass Index and Serological Responses to SARS-CoV-2 Vaccination in Patients With Inflammatory Bowel Disease
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Kenneth Ernest-Suarez, MD
University of Calgary
Calgary, AB, Canada
Presenting Author(s)
Kenneth Ernest-Suarez, MD1, Fiona Yeaman, MBBS1, Joshua Quan, MSc1, Catherine Rowan, MD1, Christopher Ma, MD2, Remo Panaccione, MD1, Lindsay Hracs, PhD1, Nastaran Sharifi, MD1, Michelle Herauf, MSc1, Ante Markovinović, BA1, Stephanie Coward, PhD1, Joseph W.. Windsor, PhD1, Léa Caplan, BHSc1, Richard J. M.. Ingram, MD, PhD1, Cynthia Seow, MD1, Kerri Novak, MD1, Cathy Lu, MD, MSc1, Gilaad G. Kaplan, MD, MPH1
1University of Calgary, Calgary, AB, Canada; 2Alimentiv Inc., University of Calgary, London, ON, Canada
Introduction: Vaccines have been shown effective regardless of Body Mass Index (BMI), obesity may impact antibody levels compared to healthy weight subjects. The durability of vaccine response in people with obesity has not been definitively studied in those IBD. This study aims to determine the association between elevated BMI and serological responses (SR) to SARS-CoV-2 vaccination in individuals with IBD.
Methods: SARS-CoV-2 vaccinated (≥ 2 doses) adults with IBD were recruited from the STOP COVID-19 IBD cohort. Patient height and weight recorded at recruitment were used to calculate BMI. Individuals were stratified into “obese” (BMI ≥30) and “normal to overweight” (BMI 18.5-30) groups according to the CDC Obesity classification. IgG antibodies to the spike protein of SARS-CoV-2 (anti-S) were assessed using the Abbott Architect SARS-CoV-2 IgG II Quant assay at 1–8 weeks after 1st dose vaccination, and 1–8 weeks and 8+ weeks after 2nd, 3rd, and 4th dose vaccination. Sex, age, IBD type, and medication status at 1st dose of vaccine were collected via chart review and compared between BMI groups using Chi-square tests for frequencies or Mann-Whitney U test for means. Positive SR (≥50 AU/mL) rates were compared between BMI groups using two-sample proportion tests. Anti-S concentrations, reported as geometric mean titres (GMT), were compared using Mann Whitney-U tests. For timepoints with significant GMT differences, multivariable linear regression was used to model the association between obesity and anti-S concentration, adjusted for age, sex, IBD type, medication class, and prior COVID-19 infection.
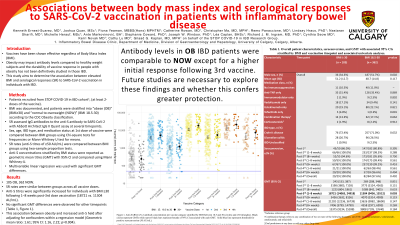
Results: 105 individuals have with obesity and 362 non-obese were recruited. SR rates were similar between groups across all vaccine doses. Anti-S titres were significantly increased for individuals with BMI ≥30 following 1–8 weeks post-3rd dose vaccination (18721 vs. 11304 AU/mL). However, no significant GMT differences were observed for other timepoints (Table 1, Figure 1). This association between obesity and increased anti-S held after adjusting for confounders within a regression model (Geometric mean ratio: 1.61; 95% CI: 1.16, 2.22; p=0.004).
Discussion: Antibody levels in obese IBD patients were similar to non-obese with the exception of a higher initial response following 3rd vaccine dose. Future studies are necessary to explore clinical, pharmacodynamic, or immunological factors explaining a higher SR in obese individuals with IBD, and whether this confers greater protection.

Disclosures:
Kenneth Ernest-Suarez, MD1, Fiona Yeaman, MBBS1, Joshua Quan, MSc1, Catherine Rowan, MD1, Christopher Ma, MD2, Remo Panaccione, MD1, Lindsay Hracs, PhD1, Nastaran Sharifi, MD1, Michelle Herauf, MSc1, Ante Markovinović, BA1, Stephanie Coward, PhD1, Joseph W.. Windsor, PhD1, Léa Caplan, BHSc1, Richard J. M.. Ingram, MD, PhD1, Cynthia Seow, MD1, Kerri Novak, MD1, Cathy Lu, MD, MSc1, Gilaad G. Kaplan, MD, MPH1. P3538 - Associations Between Body Mass Index and Serological Responses to SARS-CoV-2 Vaccination in Patients With Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Calgary, Calgary, AB, Canada; 2Alimentiv Inc., University of Calgary, London, ON, Canada
Introduction: Vaccines have been shown effective regardless of Body Mass Index (BMI), obesity may impact antibody levels compared to healthy weight subjects. The durability of vaccine response in people with obesity has not been definitively studied in those IBD. This study aims to determine the association between elevated BMI and serological responses (SR) to SARS-CoV-2 vaccination in individuals with IBD.
Methods: SARS-CoV-2 vaccinated (≥ 2 doses) adults with IBD were recruited from the STOP COVID-19 IBD cohort. Patient height and weight recorded at recruitment were used to calculate BMI. Individuals were stratified into “obese” (BMI ≥30) and “normal to overweight” (BMI 18.5-30) groups according to the CDC Obesity classification. IgG antibodies to the spike protein of SARS-CoV-2 (anti-S) were assessed using the Abbott Architect SARS-CoV-2 IgG II Quant assay at 1–8 weeks after 1st dose vaccination, and 1–8 weeks and 8+ weeks after 2nd, 3rd, and 4th dose vaccination. Sex, age, IBD type, and medication status at 1st dose of vaccine were collected via chart review and compared between BMI groups using Chi-square tests for frequencies or Mann-Whitney U test for means. Positive SR (≥50 AU/mL) rates were compared between BMI groups using two-sample proportion tests. Anti-S concentrations, reported as geometric mean titres (GMT), were compared using Mann Whitney-U tests. For timepoints with significant GMT differences, multivariable linear regression was used to model the association between obesity and anti-S concentration, adjusted for age, sex, IBD type, medication class, and prior COVID-19 infection.
Results: 105 individuals have with obesity and 362 non-obese were recruited. SR rates were similar between groups across all vaccine doses. Anti-S titres were significantly increased for individuals with BMI ≥30 following 1–8 weeks post-3rd dose vaccination (18721 vs. 11304 AU/mL). However, no significant GMT differences were observed for other timepoints (Table 1, Figure 1). This association between obesity and increased anti-S held after adjusting for confounders within a regression model (Geometric mean ratio: 1.61; 95% CI: 1.16, 2.22; p=0.004).
Discussion: Antibody levels in obese IBD patients were similar to non-obese with the exception of a higher initial response following 3rd vaccine dose. Future studies are necessary to explore clinical, pharmacodynamic, or immunological factors explaining a higher SR in obese individuals with IBD, and whether this confers greater protection.

Figure: Figure 1. Anti-SARS-CoV-2 antibody concentration per vaccine category stratified by BMI between 18.5 and 30 (circles) and ≥30 (triangles). Black circles represent GMTs while narrow black bars represent bounds of 95% CI associated with each GMT. Solid blue line represents threshold for positive seroconversion (50 AU/mL).
Disclosures:
Kenneth Ernest-Suarez: AstraZeneca – Advisor or Review Panel Member, Consultant. Ferring – Advisor or Review Panel Member, Consultant. Janssen – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Sandoz – Advisor or Review Panel Member, Consultant.
Fiona Yeaman indicated no relevant financial relationships.
Joshua Quan indicated no relevant financial relationships.
Catherine Rowan indicated no relevant financial relationships.
Christopher Ma: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. AVIR Pharma Inc – Consultant, Speakers Bureau. BioJAMP – Consultant. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant. Ferring – Consultant, Grant/Research Support, Speakers Bureau. Fresenius Kabi – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. McKesson – Consultant. Mylan – Consultant. Pendopharm – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prometheus Biosciences Inc. – Consultant. Roche – Consultant. Sanofi – Consultant. Springer Publishing – Royalties. Takeda – Consultant, Speakers Bureau.
Remo Panaccione: Abbivax – Consultant. Abbott – Consultant. AbbVie – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena – Consultant. AstraZeneca – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion – Consultant. Cosmos Technology – Consultant. Eisai – Consultant. Elan – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. JAMP Bio – Consultant. Janssen – Consultant. Merck – Consultant. Mylan – Consultant. Novartis – Consultant. Oppilan Pharma – Consultant. Organon – Consultant. Pandion Pharma – Consultant. Pendopharm – Consultant. Pfizer Inc – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist Therapeutics – Consultant. Roche – Consultant. Sandoz – Consultant. Satisfai Health – Consultant. Shire – Consultant. Sublimity Therapeutics – Consultant. Takeda Pharmaceuticals – Consultant. Theravance Biopharma – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Consultant. Viatris – Consultant.
Lindsay Hracs indicated no relevant financial relationships.
Nastaran Sharifi indicated no relevant financial relationships.
Michelle Herauf indicated no relevant financial relationships.
Ante Markovinović indicated no relevant financial relationships.
Stephanie Coward indicated no relevant financial relationships.
Joseph Windsor indicated no relevant financial relationships.
Léa Caplan indicated no relevant financial relationships.
Richard Ingram indicated no relevant financial relationships.
Cynthia Seow: Abbvie – Advisor or Review Panel Member, Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Consultant. Fresenius Kabi – Advisor or Review Panel Member, Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Kerri Novak: Abbvie – Advisor or Review Panel Member, Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Celltrion – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Janssen – Advisor or Review Panel Member, Consultant. McKesson – Advisory Committee/Board Member. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support. Takeda – Advisor or Review Panel Member, Consultant.
Cathy Lu: Abbvie – Advisory Committee/Board Member. Ferring – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member.
Gilaad Kaplan: AbbVie (received honoraria) – Consultant, Speaking. Amgen (received honoraria) – Consultant, Speaking. Ferring – Grants for research support. Janssen (received honoraria) – Consultant, Speaking. Pendophram (received honoraria) – Consultant, Speaking. Pfizer (received honoraria) – Consultant, Speaking. Sandoz (received honoraria) – Consultant, Speaking. Takeda (received honoraria) – Consultant, Speaking. TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. 7 Sept. – Intellectual Property/Patents.
Kenneth Ernest-Suarez, MD1, Fiona Yeaman, MBBS1, Joshua Quan, MSc1, Catherine Rowan, MD1, Christopher Ma, MD2, Remo Panaccione, MD1, Lindsay Hracs, PhD1, Nastaran Sharifi, MD1, Michelle Herauf, MSc1, Ante Markovinović, BA1, Stephanie Coward, PhD1, Joseph W.. Windsor, PhD1, Léa Caplan, BHSc1, Richard J. M.. Ingram, MD, PhD1, Cynthia Seow, MD1, Kerri Novak, MD1, Cathy Lu, MD, MSc1, Gilaad G. Kaplan, MD, MPH1. P3538 - Associations Between Body Mass Index and Serological Responses to SARS-CoV-2 Vaccination in Patients With Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
