Tuesday Poster Session
Category: IBD
P3554 - An Assessment of Ustekinumab Immunogenicity in Perianal Crohn’s Disease
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Scott Manski, MD
Thomas Jefferson University Hospital
Philadelphia, PA
Presenting Author(s)
Scott Manski, MD, Vincent Dioguardi, MD, Blake J. Weil, BA, Chelsea Edirisuriya, MD, Raina Shivashankar, MD, FACG
Thomas Jefferson University Hospital, Philadelphia, PA
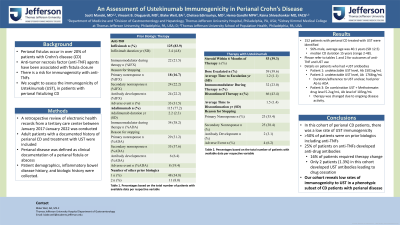
Introduction: Perianal fistulas occur in over 20% of patients with Crohn’s disease (CD). While anti-tumor necrosis factor (anti-TNF) agents have been associated with fistula closure, they are also associated with immunogenicity. Here, we sought to assess the immunogenicity of a novel biologic, Ustekinumab (UST), in patients with perianal fistulizing CD.
Methods: A retrospective review of electronic health records from a tertiary care center between January 2017-January 2022 was conducted. Adult patients with a documented history of perianal CD and treatment with UST were included. Perianal disease was defined as clinical documentation of a perianal fistula or abscess. Patient demographics, inflammatory bowel disease history, and biologic history were collected.
Results: We identified 152 patients with perianal CD who were treated with UST; 56% were male. Average age was 40.1 years (SD 12.5) and median CD duration was 15 years (range 2-48). Eighty-four percent of patients had previously been on infliximab (IFX) and 77% on adalimumab (ADA) with an average duration of 3.2 years (SD 4.7). Therapy was changed due to antibody formation in 22.2% of IFX-treated patients and in 6.4% of ADA-treated patients. While on UST, 59% required dose escalation; 23% were also treated with an immunomodulator. Although 56.6% of patients continued UST therapy, the most common indications for cessation were primary (35%) and secondary (38%) nonresponse. Two patients changed therapy due to undetectable UST levels with antibody levels of 1501ng/mL and 1768ng/mL. One patient was on UST for < 1 year due to antibody development and had prior antibodies to IFX and ADA. In the 2nd patient, the duration of and adherence to UST was unclear; they had prior antibodies to ADA. A 3rd patient on UST and methotrexate had an antibody level of 103ng/mL with a drug level of 5.2ug/mL after 2.6 years on therapy. Their therapy was changed shortly after due to ongoing disease activity.
Discussion: In this cohort of perianal CD patients, low rates of immunogenicity to UST were found. Over 50% of patients were on prior biologics including anti-TNFs. While 25% of patients developed anti-TNF antibodies with 16% of patients changing therapy due to antibody formation, clinically significant UST antibodies leading to drug cessation were seen in only 2 patients (1.3% of our cohort). This study provides additional evidence for low rates of immunogenicity to UST in a phenotypic subset of Crohn’s patients with perianal disease.
Disclosures:
Scott Manski, MD, Vincent Dioguardi, MD, Blake J. Weil, BA, Chelsea Edirisuriya, MD, Raina Shivashankar, MD, FACG. P3554 - An Assessment of Ustekinumab Immunogenicity in Perianal Crohn’s Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Thomas Jefferson University Hospital, Philadelphia, PA
Introduction: Perianal fistulas occur in over 20% of patients with Crohn’s disease (CD). While anti-tumor necrosis factor (anti-TNF) agents have been associated with fistula closure, they are also associated with immunogenicity. Here, we sought to assess the immunogenicity of a novel biologic, Ustekinumab (UST), in patients with perianal fistulizing CD.
Methods: A retrospective review of electronic health records from a tertiary care center between January 2017-January 2022 was conducted. Adult patients with a documented history of perianal CD and treatment with UST were included. Perianal disease was defined as clinical documentation of a perianal fistula or abscess. Patient demographics, inflammatory bowel disease history, and biologic history were collected.
Results: We identified 152 patients with perianal CD who were treated with UST; 56% were male. Average age was 40.1 years (SD 12.5) and median CD duration was 15 years (range 2-48). Eighty-four percent of patients had previously been on infliximab (IFX) and 77% on adalimumab (ADA) with an average duration of 3.2 years (SD 4.7). Therapy was changed due to antibody formation in 22.2% of IFX-treated patients and in 6.4% of ADA-treated patients. While on UST, 59% required dose escalation; 23% were also treated with an immunomodulator. Although 56.6% of patients continued UST therapy, the most common indications for cessation were primary (35%) and secondary (38%) nonresponse. Two patients changed therapy due to undetectable UST levels with antibody levels of 1501ng/mL and 1768ng/mL. One patient was on UST for < 1 year due to antibody development and had prior antibodies to IFX and ADA. In the 2nd patient, the duration of and adherence to UST was unclear; they had prior antibodies to ADA. A 3rd patient on UST and methotrexate had an antibody level of 103ng/mL with a drug level of 5.2ug/mL after 2.6 years on therapy. Their therapy was changed shortly after due to ongoing disease activity.
Discussion: In this cohort of perianal CD patients, low rates of immunogenicity to UST were found. Over 50% of patients were on prior biologics including anti-TNFs. While 25% of patients developed anti-TNF antibodies with 16% of patients changing therapy due to antibody formation, clinically significant UST antibodies leading to drug cessation were seen in only 2 patients (1.3% of our cohort). This study provides additional evidence for low rates of immunogenicity to UST in a phenotypic subset of Crohn’s patients with perianal disease.
Disclosures:
Scott Manski indicated no relevant financial relationships.
Vincent Dioguardi indicated no relevant financial relationships.
Blake Weil indicated no relevant financial relationships.
Chelsea Edirisuriya indicated no relevant financial relationships.
Raina Shivashankar: Abbvie – Grant/Research Support, Speakers Bureau. Bristol Meyers Squibb – Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support.
Scott Manski, MD, Vincent Dioguardi, MD, Blake J. Weil, BA, Chelsea Edirisuriya, MD, Raina Shivashankar, MD, FACG. P3554 - An Assessment of Ustekinumab Immunogenicity in Perianal Crohn’s Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
