Tuesday Poster Session
Category: IBD
P3556 - Real-World Clinical, Endoscopic, and Safety Outcomes After Upadacitinib Induction for Ulcerative Colitis: A Multicenter Retrospective Cohort Study
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- RD
Rahul Dalal, MD, MPH
Brigham and Women's Hospital, Harvard Medical School
Boston, MA
Presenting Author(s)
Rahul Dalal, MD, MPH1, Govind Kallumkal, MD2, Heidy Cabral, BS, MD1, Salam Bachour, MD, MS1, Edward Barnes, MD, MPH, FACG3, Jessica R.. Allegretti, MD, MPH1
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 2University of North Carolina School of Medicine, Chapel Hill, NC; 3University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: Upadacitinib (Upa) is a novel Janus kinase-1 inhibitor approved for the treatment of moderate-to-severe ulcerative colitis (UC). We assessed real-world clinical, endoscopic, and safety outcomes after Upa induction for UC.
Methods: This retrospective cohort study included adults initiating Upa for UC between 3/1/22-2/1/23 at two large academic medical centers. Those with prior colectomy were excluded. Steroid-free clinical remission (SFCR; defined using the following criteria in order of preference based on data availability: A. simple clinical colitis activity index [SCCAI] < 2 or B. 9-point Mayo score < 2 or C. provider documentation of clinical remission), clinical response (improvement in SCCAI or Mayo score by > 3 points or provider documentation of clinical response), biochemical remission (C-reactive protein < 10 mg/L), and improvement in baseline arthralgia (if present) were assessed at 8-16 weeks after Upa initiation. Endoscopic response, endoscopic remission, drug-related adverse events (AEs), and treatment discontinuation were assessed during all available follow-up (median 34.1 weeks).
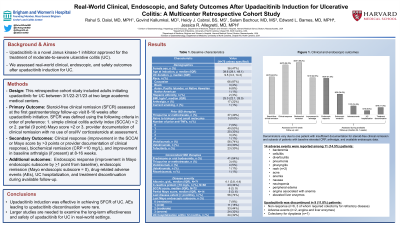
Results: Seventy-six patients initiated Upa, among which 91% had prior anti-TNF exposure, 74% had prior vedolizumab exposure, 30% had prior ustekinumab exposure, and 30% had prior tofacitinib exposure (Table 1). Among those with available data for each outcome, 64.0% (48/75) achieved SFCR, 84.2% (64/76) achieved clinical response, 88.2% (45/51) achieved biochemical remission, 62.5% (10/16) had improvement in arthralgia, 61.5% (16/26) achieved endoscopic response, and 34.6% (9/26) achieved endoscopic remission (Figure 1). Those with prior tofacitinib exposure (n=22) had similar rates of SFCR (54.5%) and clinical response (82.6%) as those without prior tofacitinib exposure (p=0.30 and p=1.00, respectively). During available follow-up, 14 AEs were reported among 11 patients: bacteremia, cellulitis, diverticulitis, pneumonia, pharyngitis, rash (n=2), acne, anemia, nausea, neutropenia, peripheral edema, angina associated with anemia, and elevated liver enzymes, Upa was discontinued in 11.8% (9/76) due to non-response (6/9, 3 of which required colectomy for refractory disease), AEs (2/9: angina and liver enzymes), and colectomy for dysplasia (1/9).
Discussion: Upa induction was effective in achieving SFCR of UC. AEs leading to Upa discontinuation were rare. Larger studies are needed to examine the long-term effectiveness and safety of Upa for UC in real-world settings.

Disclosures:
Rahul Dalal, MD, MPH1, Govind Kallumkal, MD2, Heidy Cabral, BS, MD1, Salam Bachour, MD, MS1, Edward Barnes, MD, MPH, FACG3, Jessica R.. Allegretti, MD, MPH1. P3556 - Real-World Clinical, Endoscopic, and Safety Outcomes After Upadacitinib Induction for Ulcerative Colitis: A Multicenter Retrospective Cohort Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 2University of North Carolina School of Medicine, Chapel Hill, NC; 3University of North Carolina at Chapel Hill, Chapel Hill, NC
Introduction: Upadacitinib (Upa) is a novel Janus kinase-1 inhibitor approved for the treatment of moderate-to-severe ulcerative colitis (UC). We assessed real-world clinical, endoscopic, and safety outcomes after Upa induction for UC.
Methods: This retrospective cohort study included adults initiating Upa for UC between 3/1/22-2/1/23 at two large academic medical centers. Those with prior colectomy were excluded. Steroid-free clinical remission (SFCR; defined using the following criteria in order of preference based on data availability: A. simple clinical colitis activity index [SCCAI] < 2 or B. 9-point Mayo score < 2 or C. provider documentation of clinical remission), clinical response (improvement in SCCAI or Mayo score by > 3 points or provider documentation of clinical response), biochemical remission (C-reactive protein < 10 mg/L), and improvement in baseline arthralgia (if present) were assessed at 8-16 weeks after Upa initiation. Endoscopic response, endoscopic remission, drug-related adverse events (AEs), and treatment discontinuation were assessed during all available follow-up (median 34.1 weeks).
Results: Seventy-six patients initiated Upa, among which 91% had prior anti-TNF exposure, 74% had prior vedolizumab exposure, 30% had prior ustekinumab exposure, and 30% had prior tofacitinib exposure (Table 1). Among those with available data for each outcome, 64.0% (48/75) achieved SFCR, 84.2% (64/76) achieved clinical response, 88.2% (45/51) achieved biochemical remission, 62.5% (10/16) had improvement in arthralgia, 61.5% (16/26) achieved endoscopic response, and 34.6% (9/26) achieved endoscopic remission (Figure 1). Those with prior tofacitinib exposure (n=22) had similar rates of SFCR (54.5%) and clinical response (82.6%) as those without prior tofacitinib exposure (p=0.30 and p=1.00, respectively). During available follow-up, 14 AEs were reported among 11 patients: bacteremia, cellulitis, diverticulitis, pneumonia, pharyngitis, rash (n=2), acne, anemia, nausea, neutropenia, peripheral edema, angina associated with anemia, and elevated liver enzymes, Upa was discontinued in 11.8% (9/76) due to non-response (6/9, 3 of which required colectomy for refractory disease), AEs (2/9: angina and liver enzymes), and colectomy for dysplasia (1/9).
Discussion: Upa induction was effective in achieving SFCR of UC. AEs leading to Upa discontinuation were rare. Larger studies are needed to examine the long-term effectiveness and safety of Upa for UC in real-world settings.

Figure: Figure 1. Clinical and endoscopic outcomes. Denominators vary due to one patient with insufficient documentation for steroid-free clinical remission and subsets of patients with baseline elevated CRP, arthralgia, and available endoscopic data.
Disclosures:
Rahul Dalal: Centaur Labs – Consultant. Janssen – Consultant, Grant/Research Support. Pfizer – Grant/Research Support.
Govind Kallumkal indicated no relevant financial relationships.
Heidy Cabral indicated no relevant financial relationships.
Salam Bachour indicated no relevant financial relationships.
Edward Barnes: AbbVie, Inc. – Consultant. Bristol-Meyers Squibb – Consultant. Eli Lilly – Consultant. Target RWE – Consultant.
Jessica Allegretti: Abbvie – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Artizan – Consultant. Artugen Therapeutics – Consultant. Baccain – Consultant. Bristol-Myers Squibb/Celgene – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant. Finch Therapeutics – Consultant. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Merck – Consultant, Grant/Research Support. Morphic – Consultant. Pandion Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Roivant Sciences – Consultant. Seres Therapeutics – Consultant. Servatus – Consultant. Summit – Consultant.
Rahul Dalal, MD, MPH1, Govind Kallumkal, MD2, Heidy Cabral, BS, MD1, Salam Bachour, MD, MS1, Edward Barnes, MD, MPH, FACG3, Jessica R.. Allegretti, MD, MPH1. P3556 - Real-World Clinical, Endoscopic, and Safety Outcomes After Upadacitinib Induction for Ulcerative Colitis: A Multicenter Retrospective Cohort Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
