Tuesday Poster Session
Category: IBD
P3571 - Impact of Ozanimod on Interleukin-17A Levels and the Association With OZA Efficacy in Patients With Moderately to Severely Active Ulcerative Colitis : Data From the Phase 3 True North Study
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
- SH
Sarah Harris, PhD
Bristol Myers Squibb
Princeton, New Jersey
Presenting Author(s)
Sarah Harris, PhD1, Rachel Maddux, PhD2, Chun Wu, PhD1, Yanhua Hu, PhD1, AnnKatrin Petersen, MD1, Séverine Vermeire, MD, PhD3
1Bristol Myers Squibb, Princeton, NJ; 2Bristol Myers Squibb, Princeton, PA; 3UZ Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: OZA was approved for the treatment of moderately to severely active UC based on results from the TN study. IL-17A, a proinflammatory cytokine, sustains the release of other inflammatory mediators and is associated with inflammatory bowel disease.
Methods: This analysis assessed the effect of OZA on IL-17A levels and the association of IL-17A levels with OZA efficacy during TN. During the 10-wk induction period, pts in Cohort 1 were randomized to OZA 0.92 mg or placebo (PBO) and pts in Cohort 2 received open-label OZA 0.92 mg. OZA clinical responders at Week (W) 10 were rerandomized to OZA (OZA/OZA pts) or PBO (OZA/PBO pts) for maintenance through W52. Associations of IL-17A levels with disease activity (rectal bleeding, stool frequency, Physician’s Global Assessment, and endoscopy subscores and partial and total Mayo scores) and efficacy endpoints (clinical remission, clinical response, endoscopic improvement, mucosal healing, and histologic remission) were assessed using Spearman’s correlation and logistic regression, respectively.
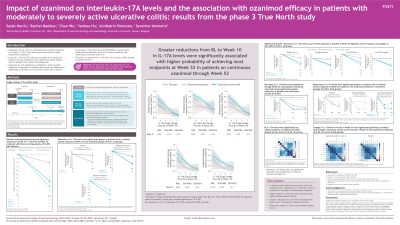
Results: Pts on OZA demonstrated significant reductions from baseline in IL-17A at W10 vs PBO (P< 0.001); IL-17A reductions were significantly greater in pts with vs without clinical response at W10 in all OZA and PBO treatment groups (P< 0.01) (Table). IL-17A reductions were maintained through W52; OZA/OZA pts had significantly greater IL-17A reductions at W52 vs OZA/PBO pts (P< 0.001); IL-17A reductions were significantly greater in pts with vs without clinical response at W52 (P< 0.001, OZA and PBO groups) (Table). Baseline IL-17A levels were significantly correlated with all baseline disease activity scores (Spearman’s rho 0.1–0.2, P< 0.05), but were not associated with treatment responses at W10. Change in IL-17A from baseline to W10 was significantly correlated with change in all disease activity scores at W10 with OZA and PBO (Spearman’s rho 0.2–0.4, P< 0.05). Furthermore, greater reductions from baseline to W10 in IL-17A was significantly associated with higher probability of achieving clinical remission, clinical response, endoscopic improvement, and mucosal healing at W52 (P< 0.05) in OZA/OZA pts through W52.
Discussion: OZA led to IL-17A reductions, indicative of decreases in inflammatory responses. Higher baseline IL-17A was associated with higher baseline disease activity, and greater reductions in IL-17A were associated with better short- and long-term disease outcomes, supporting IL-17A as a good disease marker for UC.
Disclosures:
Sarah Harris, PhD1, Rachel Maddux, PhD2, Chun Wu, PhD1, Yanhua Hu, PhD1, AnnKatrin Petersen, MD1, Séverine Vermeire, MD, PhD3. P3571 - Impact of Ozanimod on Interleukin-17A Levels and the Association With OZA Efficacy in Patients With Moderately to Severely Active Ulcerative Colitis : Data From the Phase 3 True North Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Bristol Myers Squibb, Princeton, NJ; 2Bristol Myers Squibb, Princeton, PA; 3UZ Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: OZA was approved for the treatment of moderately to severely active UC based on results from the TN study. IL-17A, a proinflammatory cytokine, sustains the release of other inflammatory mediators and is associated with inflammatory bowel disease.
Methods: This analysis assessed the effect of OZA on IL-17A levels and the association of IL-17A levels with OZA efficacy during TN. During the 10-wk induction period, pts in Cohort 1 were randomized to OZA 0.92 mg or placebo (PBO) and pts in Cohort 2 received open-label OZA 0.92 mg. OZA clinical responders at Week (W) 10 were rerandomized to OZA (OZA/OZA pts) or PBO (OZA/PBO pts) for maintenance through W52. Associations of IL-17A levels with disease activity (rectal bleeding, stool frequency, Physician’s Global Assessment, and endoscopy subscores and partial and total Mayo scores) and efficacy endpoints (clinical remission, clinical response, endoscopic improvement, mucosal healing, and histologic remission) were assessed using Spearman’s correlation and logistic regression, respectively.
Results: Pts on OZA demonstrated significant reductions from baseline in IL-17A at W10 vs PBO (P< 0.001); IL-17A reductions were significantly greater in pts with vs without clinical response at W10 in all OZA and PBO treatment groups (P< 0.01) (Table). IL-17A reductions were maintained through W52; OZA/OZA pts had significantly greater IL-17A reductions at W52 vs OZA/PBO pts (P< 0.001); IL-17A reductions were significantly greater in pts with vs without clinical response at W52 (P< 0.001, OZA and PBO groups) (Table). Baseline IL-17A levels were significantly correlated with all baseline disease activity scores (Spearman’s rho 0.1–0.2, P< 0.05), but were not associated with treatment responses at W10. Change in IL-17A from baseline to W10 was significantly correlated with change in all disease activity scores at W10 with OZA and PBO (Spearman’s rho 0.2–0.4, P< 0.05). Furthermore, greater reductions from baseline to W10 in IL-17A was significantly associated with higher probability of achieving clinical remission, clinical response, endoscopic improvement, and mucosal healing at W52 (P< 0.05) in OZA/OZA pts through W52.
Discussion: OZA led to IL-17A reductions, indicative of decreases in inflammatory responses. Higher baseline IL-17A was associated with higher baseline disease activity, and greater reductions in IL-17A were associated with better short- and long-term disease outcomes, supporting IL-17A as a good disease marker for UC.
Disclosures:
Sarah Harris: Bristol Myers Squibb – employee and/or shareholder.
Rachel Maddux: Bristol Myers Squibb – employee and/or shareholder.
Chun Wu: Bristol Myers Squibb – employee and/or shareholder.
Yanhua Hu: Bristol Myers Squibb – employee and/or shareholder.
AnnKatrin Petersen: Bristol Myers Squibb – employee and/or shareholder.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. AbolerIS Pharma – Grant/Research Support. AgomAb – Grant/Research Support. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. Avaxia Biologics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech/Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIDomics – Consultant, Speakers Bureau. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. ProDigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillotts Pharma – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Sarah Harris, PhD1, Rachel Maddux, PhD2, Chun Wu, PhD1, Yanhua Hu, PhD1, AnnKatrin Petersen, MD1, Séverine Vermeire, MD, PhD3. P3571 - Impact of Ozanimod on Interleukin-17A Levels and the Association With OZA Efficacy in Patients With Moderately to Severely Active Ulcerative Colitis : Data From the Phase 3 True North Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
