Tuesday Poster Session
Category: IBD
P3572 - Association of Nonswitched Memory B-Cell (MBC) Levels With Ozanimod (OZA) Efficacy in Patients (Pts) With Moderately to Severely Active Crohn’s Disease (CD): Results From the Phase 2 STEPSTONE Study
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
- SH
Sarah Harris, PhD
Bristol Myers Squibb
Princeton, New Jersey
Presenting Author(s)
Sarah Harris, PhD1, Brian G. Feagan, MD2, Stephen B.. Hanauer, MD, FACG3, Séverine Vermeire, MD, PhD4, Subrata Ghosh, 5, Jim Yan, PhD6, Chun Wu, PhD1, Yanhua Hu, PhD1, Rachel Maddux, PhD7, Douglas C. Wolf, MD, FACG8, Geert R. D’Haens, PhD9
1Bristol Myers Squibb, Princeton, NJ; 2Western University, London, ON, Canada; 3Northwestern University Feinberg School of Medicine, Chicago, IL; 4UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 5APC Microbiome Ireland, College of Medicine and Health, University College, Cork, Cork, Ireland; 6Laboratory Corporation of America, Durham, NC; 7Bristol Myers Squibb, Princeton, PA; 8Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 9Amsterdam University Medical Center, Amsterdam, Drenthe, Netherlands
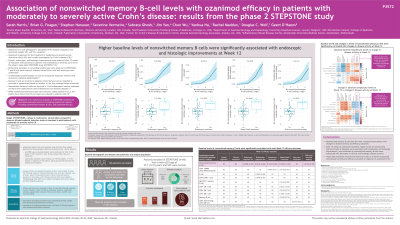
Introduction: OZA is approved for the treatment (tx) of moderately to severely active ulcerative colitis and is under investigation for CD. Clinical, endoscopic, and histologic improvements were observed after 12wk of tx with OZA in pts with moderately to severely active CD in the phase 2 STEPSTONE study.
Methods: This exploratory analysis of STEPSTONE assessed the association between levels of circulating lymphocyte subsets and OZA efficacy. Pts received OZA 0.92 mg for 12wk. Lymphocyte subsets were evaluated using multicolor flow analysis on blood samples collected before tx and at Week (W) 12; differences between Day 1 and W12 were analyzed by Wilcoxon signed-rank tests. Change in disease activity from baseline (BL) to W12 was determined for Simple Endoscopic Score for Crohn’s Disease, size of ulcers, extent of ulcerated surface, extent of affected surface, presence of narrowing, Crohn’s Disease Activity Index (CDAI), Robart’s Histopathology Index (RHI), Geboes Histology Activity Score (GHAS), and stool frequency. Efficacy outcomes were evaluated at W12. Associations of lymphocyte subset levels with disease activity changes and efficacy outcomes were assessed using Spearman’s correlation and logistic regression, respectively.
Results: Of 69 pts enrolled, 52 had disease activity, efficacy outcome, and flow cytometry data at BL and W12 and were included in this analysis. Most lymphocyte subsets assessed were significantly associated with 1 or 2 efficacy outcomes. Nonswitched MBCs were significantly associated with most outcomes, including endoscopic response-25, endoscopic response-50, endoscopic remission, global GHAS remission, and RHI mucosal healing (Table). BL levels of nonswitched MBCs were significantly correlated with change from BL to W12 in extent of affected surface (Spearman’s rho [SR] = –0.44, P< 0.05), RHI (SR = –0.34, P< 0.05), and GHAS (SR = –0.47, P< 0.05). Changes in levels of nonswitched MBCs were significantly correlated with change from BL to W12 in CDAI (SR = 0.38, P< 0.05), RHI (SR = 0.37, P< 0.05), GHAS (SR = 0.43, P< 0.05), and stool frequency (SR = 0.43, P< 0.05).
Discussion: After 12wk of OZA tx, higher levels of nonswitched MBCs at BL were associated with endoscopic and histologic improvement, and reductions in nonswitched MBCs were associated with decreases in clinical and histologic disease activity. These data implicate nonswitched MBCs, which are involved in T cell–dependent immune responses and some inflammatory diseases, as a marker associated with OZA response in CD.
Disclosures:
Sarah Harris, PhD1, Brian G. Feagan, MD2, Stephen B.. Hanauer, MD, FACG3, Séverine Vermeire, MD, PhD4, Subrata Ghosh, 5, Jim Yan, PhD6, Chun Wu, PhD1, Yanhua Hu, PhD1, Rachel Maddux, PhD7, Douglas C. Wolf, MD, FACG8, Geert R. D’Haens, PhD9. P3572 - Association of Nonswitched Memory B-Cell (MBC) Levels With Ozanimod (OZA) Efficacy in Patients (Pts) With Moderately to Severely Active Crohn’s Disease (CD): Results From the Phase 2 STEPSTONE Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Bristol Myers Squibb, Princeton, NJ; 2Western University, London, ON, Canada; 3Northwestern University Feinberg School of Medicine, Chicago, IL; 4UZ Leuven, Leuven, Vlaams-Brabant, Belgium; 5APC Microbiome Ireland, College of Medicine and Health, University College, Cork, Cork, Ireland; 6Laboratory Corporation of America, Durham, NC; 7Bristol Myers Squibb, Princeton, PA; 8Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 9Amsterdam University Medical Center, Amsterdam, Drenthe, Netherlands
Introduction: OZA is approved for the treatment (tx) of moderately to severely active ulcerative colitis and is under investigation for CD. Clinical, endoscopic, and histologic improvements were observed after 12wk of tx with OZA in pts with moderately to severely active CD in the phase 2 STEPSTONE study.
Methods: This exploratory analysis of STEPSTONE assessed the association between levels of circulating lymphocyte subsets and OZA efficacy. Pts received OZA 0.92 mg for 12wk. Lymphocyte subsets were evaluated using multicolor flow analysis on blood samples collected before tx and at Week (W) 12; differences between Day 1 and W12 were analyzed by Wilcoxon signed-rank tests. Change in disease activity from baseline (BL) to W12 was determined for Simple Endoscopic Score for Crohn’s Disease, size of ulcers, extent of ulcerated surface, extent of affected surface, presence of narrowing, Crohn’s Disease Activity Index (CDAI), Robart’s Histopathology Index (RHI), Geboes Histology Activity Score (GHAS), and stool frequency. Efficacy outcomes were evaluated at W12. Associations of lymphocyte subset levels with disease activity changes and efficacy outcomes were assessed using Spearman’s correlation and logistic regression, respectively.
Results: Of 69 pts enrolled, 52 had disease activity, efficacy outcome, and flow cytometry data at BL and W12 and were included in this analysis. Most lymphocyte subsets assessed were significantly associated with 1 or 2 efficacy outcomes. Nonswitched MBCs were significantly associated with most outcomes, including endoscopic response-25, endoscopic response-50, endoscopic remission, global GHAS remission, and RHI mucosal healing (Table). BL levels of nonswitched MBCs were significantly correlated with change from BL to W12 in extent of affected surface (Spearman’s rho [SR] = –0.44, P< 0.05), RHI (SR = –0.34, P< 0.05), and GHAS (SR = –0.47, P< 0.05). Changes in levels of nonswitched MBCs were significantly correlated with change from BL to W12 in CDAI (SR = 0.38, P< 0.05), RHI (SR = 0.37, P< 0.05), GHAS (SR = 0.43, P< 0.05), and stool frequency (SR = 0.43, P< 0.05).
Discussion: After 12wk of OZA tx, higher levels of nonswitched MBCs at BL were associated with endoscopic and histologic improvement, and reductions in nonswitched MBCs were associated with decreases in clinical and histologic disease activity. These data implicate nonswitched MBCs, which are involved in T cell–dependent immune responses and some inflammatory diseases, as a marker associated with OZA response in CD.
Disclosures:
Sarah Harris: Bristol Myers Squibb – employee and/or shareholder.
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Consultant. Applied Molecular Transport Inc. – Consultant. Arena Pharma – Consultant. Avir – Consultant. Azora Therapeutics – Consultant. Baxter – Consultant. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boston Pharma – Consultant. Bristol Myers Squibb – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Connect BioPharma – Consultant. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 – Advisory Committee/Board Member, Consultant. Eli Lilly – Consultant. Equillium – Consultant. Ermium – Consultant. Everest Clinical Research Corp. – Consultant. Ferring Pharmaceuticals – Consultant. First Wave – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Glenmark – Consultant. Gossamer Pharma – Consultant, stock shareholder. Hoffmann-LaRoche – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. ImmunExt – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Advisory Committee/Board Member, Consultant. Intact Therapeutics – Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Consultant. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. Novartis – Advisory Committee/Board Member. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Otsuka – Consultant. Pandion Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Therapeutics and Diagnostics – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. RedHill Biopharma – Consultant. Redx – Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Theravance – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. UCB Pharma – Consultant. VHSquared Ltd. – Consultant. Viatris – Consultant. Western University, Alimentiv Inc – Employee. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Stephen Hanauer: AbbVie – Consultant, Speakers Bureau. Allergan – Advisor or Review Panel Member, Consultant. Amgen – Consultant. arena – Advisor or Review Panel Member, Consultant. BMS – Advisor or Review Panel Member, Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, participating in Data and Safety Monitoring Boards. Celgene – Consultant. Celltrion – Consultant. Fresenius Kabi – Consultant. Genentech – Consultant, Grant/Research Support. Gilead – Consultant, Grant/Research Support. Gossamer – Advisor or Review Panel Member. GSK – Consultant, Grant/Research Support. InDex – Advisor or Review Panel Member. Intercept – Advisor or Review Panel Member. Janssen – Consultant, Speakers Bureau. Lilly – Consultant, Speakers Bureau. Merck – Consultant. Morphic – Consultant. Novartis – Consultant. Pfizer – Consultant, Speakers Bureau. Progenity – Consultant. Prometheus – Advisor or Review Panel Member, Consultant, Grant/Research Support. Protagonist – Advisor or Review Panel Member, Consultant. Salix – Consultant. Samsung Bioepis – Consultant. Seres – Consultant. Takeda – Consultant, Grant/Research Support, Speakers Bureau. TTL Pharma – Consultant. Ventyx – Advisor or Review Panel Member.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. AbolerIS Pharma – Grant/Research Support. AgomAb – Grant/Research Support. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. Avaxia Biologics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech/Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIDomics – Consultant, Speakers Bureau. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. ProDigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillotts Pharma – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Subrata Ghosh: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Bristol-Myers Squibb – Consultant. Celgene – Speakers Bureau. Eli Lilly – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Gilead – Speakers Bureau. GlaxoSmithKline – Grant/Research Support. Janssen – Consultant, Speakers Bureau. MSD – Speakers Bureau. Novo Nordisk – Consultant. Pfizer – Consultant, Speakers Bureau. Roche – Consultant. Takeda – Consultant, Speakers Bureau.
Jim Yan: Laboratory Corporation of America Holdings – Employee, shareholder.
Chun Wu: Bristol Myers Squibb – employee and/or shareholder.
Yanhua Hu: Bristol Myers Squibb – employee and/or shareholder.
Rachel Maddux: Bristol Myers Squibb – employee and/or shareholder.
Douglas C. Wolf: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Grant/Research Support. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant. Genentech – Grant/Research Support. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant. Pfizer/Arena – Grant/Research Support. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Geert D’Haens: AbbVie – Advisor or Review Panel Member, Speakers Bureau. Agomab Therapeutics – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. Applied Molecular Therapeutics – Advisor or Review Panel Member. AstraZeneca – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Cytoki – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member, Speakers Bureau. Exeliom – Advisor or Review Panel Member. Ferring – Advisor or Review Panel Member. Galapagos – Advisor or Review Panel Member, Speakers Bureau. GlaxoSmithKline – Advisor or Review Panel Member. Gossamer Bio – Advisor or Review Panel Member. Immunic – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Speakers Bureau. Polpharma – Advisor or Review Panel Member. ProciseDx – Advisor or Review Panel Member. Progenity – Advisor or Review Panel Member. Prometheus Biosciences – Advisor or Review Panel Member. Prometheus Laboratories – Advisor or Review Panel Member. Protagonist Therapeutics – Advisor or Review Panel Member. Seres – Advisor or Review Panel Member. Takeda – Advisor or Review Panel Member, Speakers Bureau. Tillotts – Advisor or Review Panel Member, Speakers Bureau. Versant – Advisor or Review Panel Member.
Sarah Harris, PhD1, Brian G. Feagan, MD2, Stephen B.. Hanauer, MD, FACG3, Séverine Vermeire, MD, PhD4, Subrata Ghosh, 5, Jim Yan, PhD6, Chun Wu, PhD1, Yanhua Hu, PhD1, Rachel Maddux, PhD7, Douglas C. Wolf, MD, FACG8, Geert R. D’Haens, PhD9. P3572 - Association of Nonswitched Memory B-Cell (MBC) Levels With Ozanimod (OZA) Efficacy in Patients (Pts) With Moderately to Severely Active Crohn’s Disease (CD): Results From the Phase 2 STEPSTONE Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
