Tuesday Poster Session
Category: IBD
P3581 - The Anti-TL1A Antibody PRA023 Demonstrated Proof-of-Concept in Crohn’s Disease: Phase 2a APOLLO-CD Study Results
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Brian G. Feagan, MD
Western University
London, ON, Canada
Presenting Author(s)
Award: Presidential Poster Award
Brian G. Feagan, MD1, Bruce E. Sands, MD, MS, FACG2, Corey A. Siegel, MD, MS3, Marla C. Dubinsky, MD4, Randy Longman, MD, PhD5, João Sabino, MD, PhD6, Olivier Laurent, 7, Allison Luo, MD7, JD Lu, PhD7, Deanna D.. Nguyen, MD7, Fadi Towfic, 7, Aaron DuVall, MD8, Marek Woynarowski, MD, PhD9, Houssam Al Kharrat, MD10, Dermot McGovern, MD, PhD11
1Western University, London, ON, Canada; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3Dartmouth-Hitchcock Inflammatory Bowel Disease Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 4Mount Sinai Kravis Children’s Hospital, New York, NY; 5Weill Cornell Medical College, New York, NY; 6University Hospitals Leuven, Leuven, Brussels Hoofdstedelijk Gewest, Belgium; 7Prometheus Biosciences, Inc., San Diego, CA; 8Tyler Research Institute, LLC, Tyler, TX; 9UJK Kielce, former IP CZD Warsaw, Kielce, Swietokrzyskie, Poland; 10West Texas Digestive Disease Center, Lubbock, TX; 11Cedars-Sinai Medical System, Los Angeles, CA
Introduction: Tumor necrosis factor–like cytokine 1A (TL1A) is regulator of inflammation and fibrosis. PRA023 is an anti-TL1A monoclonal antibody in development for inflammatory/fibrotic diseases with a companion genetic-based diagnostic test (Dx). This phase 2a, multicenter, open-label study aimed to assess the efficacy and safety of PRA023 as induction treatment in adults with moderately to severely active Crohn’s Disease (CD).
Methods: Key eligibility criteria included Crohn’s Disease Activity Index (CDAI) ≥220 and ≤450 with centrally read Simple Endoscopic Score (SES)-CD of ≥6 (≥4 isolated ileal disease) and a history of insufficient/loss of response and/or intolerance to conventional and/or approved biologic therapies. Eligible patients received intravenous PRA023 1000 mg on Day 1, 500 mg at weeks 2, 6, and 10. The primary endpoint was endoscopic response (reduction in SES-CD of ≥50%) at week 12. Historical placebo control rates were used as a reference for the null hypothesis. An exploratory efficacy assessment in a subpopulation identified by the Dx was included.
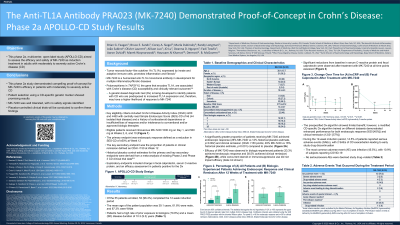
Results: Of the 55 patients enrolled, 53 (96.4%) completed the 12-week induction period. Patients had a high rate of prior biologic exposure (70.9%) and a mean (SD) disease duration of 10.3 (9.3) years (Table 1). A significantly greater proportion of patients receiving PRA023 achieved endoscopic response (26% PRA023 vs 12% historical placebo estimate, p=0.002) and clinical remission (CDAI < 150 points; 49% PRA023 vs 16% historical placebo estimate, p< 0.001). Efficacy was observed in biologic-exposed patients (33% endoscopic response and 38.5% clinical remission), while concurrent steroid or immunosuppressant use did not impact efficacy. Significant reductions from baseline in C-reactive protein and fecal calprotectin were observed at all time points (Figure 1). Although the pre-specified Dx algorithm provided only limited additional benefit in clinical responses, an alternative CD-specific algorithm demonstrated enhanced performance across both clinical (12/21 [57%] remission) and endoscopic (9/20 [45%] response) outcomes. No serious/severe adverse events were deemed as study drug–related.
Discussion: This phase 2a study demonstrated robust proof-of-concept for PRA023’s efficacy in moderately to severely active CD with favorable tolerability. Patient selection using prespecified genetic markers showed promising results. Placebo-controlled clinical trials will be conducted to confirm these findings.

Disclosures:
Brian G. Feagan, MD1, Bruce E. Sands, MD, MS, FACG2, Corey A. Siegel, MD, MS3, Marla C. Dubinsky, MD4, Randy Longman, MD, PhD5, João Sabino, MD, PhD6, Olivier Laurent, 7, Allison Luo, MD7, JD Lu, PhD7, Deanna D.. Nguyen, MD7, Fadi Towfic, 7, Aaron DuVall, MD8, Marek Woynarowski, MD, PhD9, Houssam Al Kharrat, MD10, Dermot McGovern, MD, PhD11. P3581 - The Anti-TL1A Antibody PRA023 Demonstrated Proof-of-Concept in Crohn’s Disease: Phase 2a APOLLO-CD Study Results, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Brian G. Feagan, MD1, Bruce E. Sands, MD, MS, FACG2, Corey A. Siegel, MD, MS3, Marla C. Dubinsky, MD4, Randy Longman, MD, PhD5, João Sabino, MD, PhD6, Olivier Laurent, 7, Allison Luo, MD7, JD Lu, PhD7, Deanna D.. Nguyen, MD7, Fadi Towfic, 7, Aaron DuVall, MD8, Marek Woynarowski, MD, PhD9, Houssam Al Kharrat, MD10, Dermot McGovern, MD, PhD11
1Western University, London, ON, Canada; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3Dartmouth-Hitchcock Inflammatory Bowel Disease Center, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 4Mount Sinai Kravis Children’s Hospital, New York, NY; 5Weill Cornell Medical College, New York, NY; 6University Hospitals Leuven, Leuven, Brussels Hoofdstedelijk Gewest, Belgium; 7Prometheus Biosciences, Inc., San Diego, CA; 8Tyler Research Institute, LLC, Tyler, TX; 9UJK Kielce, former IP CZD Warsaw, Kielce, Swietokrzyskie, Poland; 10West Texas Digestive Disease Center, Lubbock, TX; 11Cedars-Sinai Medical System, Los Angeles, CA
Introduction: Tumor necrosis factor–like cytokine 1A (TL1A) is regulator of inflammation and fibrosis. PRA023 is an anti-TL1A monoclonal antibody in development for inflammatory/fibrotic diseases with a companion genetic-based diagnostic test (Dx). This phase 2a, multicenter, open-label study aimed to assess the efficacy and safety of PRA023 as induction treatment in adults with moderately to severely active Crohn’s Disease (CD).
Methods: Key eligibility criteria included Crohn’s Disease Activity Index (CDAI) ≥220 and ≤450 with centrally read Simple Endoscopic Score (SES)-CD of ≥6 (≥4 isolated ileal disease) and a history of insufficient/loss of response and/or intolerance to conventional and/or approved biologic therapies. Eligible patients received intravenous PRA023 1000 mg on Day 1, 500 mg at weeks 2, 6, and 10. The primary endpoint was endoscopic response (reduction in SES-CD of ≥50%) at week 12. Historical placebo control rates were used as a reference for the null hypothesis. An exploratory efficacy assessment in a subpopulation identified by the Dx was included.
Results: Of the 55 patients enrolled, 53 (96.4%) completed the 12-week induction period. Patients had a high rate of prior biologic exposure (70.9%) and a mean (SD) disease duration of 10.3 (9.3) years (Table 1). A significantly greater proportion of patients receiving PRA023 achieved endoscopic response (26% PRA023 vs 12% historical placebo estimate, p=0.002) and clinical remission (CDAI < 150 points; 49% PRA023 vs 16% historical placebo estimate, p< 0.001). Efficacy was observed in biologic-exposed patients (33% endoscopic response and 38.5% clinical remission), while concurrent steroid or immunosuppressant use did not impact efficacy. Significant reductions from baseline in C-reactive protein and fecal calprotectin were observed at all time points (Figure 1). Although the pre-specified Dx algorithm provided only limited additional benefit in clinical responses, an alternative CD-specific algorithm demonstrated enhanced performance across both clinical (12/21 [57%] remission) and endoscopic (9/20 [45%] response) outcomes. No serious/severe adverse events were deemed as study drug–related.
Discussion: This phase 2a study demonstrated robust proof-of-concept for PRA023’s efficacy in moderately to severely active CD with favorable tolerability. Patient selection using prespecified genetic markers showed promising results. Placebo-controlled clinical trials will be conducted to confirm these findings.

Figure: Figure 1. Change Over Time in Geometric Mean Values ± SE for (A) High-Sensitivity C-Reactive Protein (hsCRP) and (B) Fecal Calprotectin
Nominal p values, * p<0.05, ** p<0.01, ***p<0.001.
Nominal p values, * p<0.05, ** p<0.01, ***p<0.001.
Disclosures:
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Consultant. Applied Molecular Transport Inc. – Consultant. Arena Pharma – Consultant. Avir – Consultant. Azora Therapeutics – Consultant. Baxter – Consultant. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boston Pharma – Consultant. Bristol Myers Squibb – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Connect BioPharma – Consultant. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 – Advisory Committee/Board Member, Consultant. Eli Lilly – Consultant. Equillium – Consultant. Ermium – Consultant. Everest Clinical Research Corp. – Consultant. Ferring Pharmaceuticals – Consultant. First Wave – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Glenmark – Consultant. Gossamer Pharma – Consultant, stock shareholder. Hoffmann-LaRoche – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. ImmunExt – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Advisory Committee/Board Member, Consultant. Intact Therapeutics – Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Consultant. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. Novartis – Advisory Committee/Board Member. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Otsuka – Consultant. Pandion Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Therapeutics and Diagnostics – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. RedHill Biopharma – Consultant. Redx – Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Theravance – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. UCB Pharma – Consultant. VHSquared Ltd. – Consultant. Viatris – Consultant. Western University, Alimentiv Inc – Employee. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speaker’s fees. Adiso Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena pharmaceuticals – Consultant. Artizan Biosciences – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees and other support. Calibr – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Connect Biopharm – Consultant. Cytoki Pharma – Consultant. Eli Lilly – Consultant, speaking fees and other support. Enthera – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. HMP Acquisition – Consultant. Imhotex – Consultant. Immunic – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Grant/Research Support, consulting and speaking fees and other support. Johnson & Johnson – Consultant. Kaleido Biosciences – Consultant. Kallyope – Consultant. Merck – Consultant. MiroBio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. OSE Immunotherapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, speaking fees and other support. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. RedHill Biopharma – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Synlogic Operating Company – Consultant. Takeda – Grant/Research Support, consulting and speaking fees and other support. Target RWE – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. Ventyx Biosciences – Consultant, personal fees and stock options for consulting. Viela Bio – Consultant.
Corey Siegel: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. BMS – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. Fresnius – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Consultant/advisory board, speaker for CME activities. Napo Pharmaceuticals – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Grant/Research Support, speaker for CME activities. Prometheus Biosciences – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena Pharmaceuticals – Consultant. Astra Zeneca – Consultant. Celgene – Consultant. Genentech Inc. – Consultant. Gilead Sciences – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Biosciences – Consultant, Grant/Research Support. Prometheus Labs – Consultant, Grant/Research Support. Takeda – Consultant, Licensing fees. Thabor – Consultant. Trellus Health – Stock-publicly held company(excluding mutual/index funds). UCB Pharma – Consultant.
Randy Longman: enzymetrics – Consultant. Pfizer – Consultant.
João Sabino: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Falk – Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Pfizer – Speakers Bureau. Pharmacosmos – Consultant. Pharmanovia – Consultant. Takeda – Speakers Bureau. Viatris – Grant/Research Support.
Olivier Laurent: Prometheus Biosciences – Employee.
Allison Luo: Prometheus Biosciences – Employee.
JD Lu: Prometheus Biosciences – Employee.
Deanna Nguyen: Prometheus Biosciences – Employee.
Fadi Towfic: Prometheus Biosciences – Employee.
Aaron DuVall indicated no relevant financial relationships.
Marek Woynarowski indicated no relevant financial relationships.
Houssam Al Kharrat indicated no relevant financial relationships.
Dermot McGovern: Boehringer-Ingelheim – Consultant. Gilead Sciences – Consultant. Palatin Technologies – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant, Stock-publicly held company(excluding mutual/index funds). Prometheus Laboratories – Consultant. Takeda – Consultant.
Brian G. Feagan, MD1, Bruce E. Sands, MD, MS, FACG2, Corey A. Siegel, MD, MS3, Marla C. Dubinsky, MD4, Randy Longman, MD, PhD5, João Sabino, MD, PhD6, Olivier Laurent, 7, Allison Luo, MD7, JD Lu, PhD7, Deanna D.. Nguyen, MD7, Fadi Towfic, 7, Aaron DuVall, MD8, Marek Woynarowski, MD, PhD9, Houssam Al Kharrat, MD10, Dermot McGovern, MD, PhD11. P3581 - The Anti-TL1A Antibody PRA023 Demonstrated Proof-of-Concept in Crohn’s Disease: Phase 2a APOLLO-CD Study Results, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

