Tuesday Poster Session
Category: IBD
P3598 - Corticosteroid-Sparing Effect of Upadacitinib in Patients With Moderately-to-Severely Active Crohn’s Disease
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio

Marla C. Dubinsky, MD
Mount Sinai Kravis Children’s Hospital
New York, NY
Presenting Author(s)
Marla C.. Dubinsky, MD1, Geert D'Haens, MD, PhD2, Olivier Dewit, MD, PhD3, Pascal Juillerat, MD, MSc4, Remo Panaccione, MD5, Toshimitsu Fujii, MD, PhD6, Ana P.. Lacerda, MD, MSc7, Elena Dubcenco, MD, MS7, Samuel I.. Anyanwu, PhD, MS7, Chirag Doshi, PharmD7, Jianzhong Liu, MD, MS7, Andrew Garrison, MS7, Edward V.. Loftus, MD8
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 3Cliniques universitaires Saint-Luc, UCLouvain, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 4Clinic for Visceral Surgery and Medicine, Bern University Hospital, Bern, Bern, Switzerland; 5University of Calgary, Calgary, AB, Canada; 6Tokyo Medical and Dental University, Tokyo, Tokyo, Japan; 7AbbVie Inc., North Chicago, IL; 8Mayo Clinic College of Medicine and Science, Rochester, MN
Introduction: Long-term use of corticosteroids (CS) in Crohn’s disease (CD) is limited by toxicities. The CS-sparing effect of upadacitinib (UPA) was evaluated among all patients with active CD in phase 3 clinical trials.
Methods: In U-EXCEL (NCT03345849) and U-EXCEED (NCT03345836), patients with moderate-to-severe CD were randomized to 12-week induction with UPA 45 mg once daily (QD) or placebo (PBO). Patients who achieved clinical response to UPA 45 mg were re‑randomized in U-ENDURE (NCT03345823) to UPA 30 mg QD, UPA 15 mg QD, or PBO for a 52-week maintenance period. Patients taking CS at baseline began a CS taper at induction week 4 and continued the taper during maintenance; those who initiated CS during the study started the CS taper on symptom improvement. Endpoints included the proportion of patients without CS use (CS-free) at week 12 or for ≥ 90 days before week 52 who achieved clinical remission by stool frequency/abdominal pain score (SF/APS) or by CD Activity Index (CDAI), enhanced clinical response, decrease of ≥ 100 points in CDAI from baseline (CR-100), endoscopic remission, and endoscopic response at week 12 and week 52. CS daily dose (in prednisone-equivalent doses) was recorded.
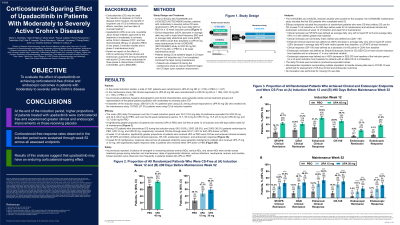
Results: Of 1021 patients evaluated, the mean (SD) cumulative CS exposure over 12 weeks was 16.0 (19.0) mg daily of prednisone equivalent for UPA 45 mg and 24.0 (34.0) mg for PBO, and during maintenance, 13.7 (16.3) mg for UPA 15 mg, 13.5 (27.3) mg for UPA 30 mg, and 13.7 (9.9) mg for PBO. A significantly higher proportion of patients who received UPA vs PBO were CS-free and achieved clinical remission (by SF/APS and CDAI), enhanced clinical response, CR-100, endoscopic remission, and endoscopic response at week 12 (Fig 1A). At week 52, response rates across all assessed endpoints were generally maintained in patients who received UPA 15 or 30 mg, with significantly higher response rates in patients who received either UPA dose vs PBO (Fig 1B). Safety was as expected for UPA or CS.1
Discussion: During induction, higher proportions of patients treated with UPA were CS-free and experienced greater clinical and endoscopic improvements vs those receiving PBO. CS‑free response rates were generally sustained through week 52, suggesting that UPA may have an enduring steroid-sparing effect.
1. Loftus EV Jr, et al. N Engl J Med. 2023;388:1966–1980.

Disclosures:
Marla C.. Dubinsky, MD1, Geert D'Haens, MD, PhD2, Olivier Dewit, MD, PhD3, Pascal Juillerat, MD, MSc4, Remo Panaccione, MD5, Toshimitsu Fujii, MD, PhD6, Ana P.. Lacerda, MD, MSc7, Elena Dubcenco, MD, MS7, Samuel I.. Anyanwu, PhD, MS7, Chirag Doshi, PharmD7, Jianzhong Liu, MD, MS7, Andrew Garrison, MS7, Edward V.. Loftus, MD8. P3598 - Corticosteroid-Sparing Effect of Upadacitinib in Patients With Moderately-to-Severely Active Crohn’s Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 3Cliniques universitaires Saint-Luc, UCLouvain, Brussels, Brussels Hoofdstedelijk Gewest, Belgium; 4Clinic for Visceral Surgery and Medicine, Bern University Hospital, Bern, Bern, Switzerland; 5University of Calgary, Calgary, AB, Canada; 6Tokyo Medical and Dental University, Tokyo, Tokyo, Japan; 7AbbVie Inc., North Chicago, IL; 8Mayo Clinic College of Medicine and Science, Rochester, MN
Introduction: Long-term use of corticosteroids (CS) in Crohn’s disease (CD) is limited by toxicities. The CS-sparing effect of upadacitinib (UPA) was evaluated among all patients with active CD in phase 3 clinical trials.
Methods: In U-EXCEL (NCT03345849) and U-EXCEED (NCT03345836), patients with moderate-to-severe CD were randomized to 12-week induction with UPA 45 mg once daily (QD) or placebo (PBO). Patients who achieved clinical response to UPA 45 mg were re‑randomized in U-ENDURE (NCT03345823) to UPA 30 mg QD, UPA 15 mg QD, or PBO for a 52-week maintenance period. Patients taking CS at baseline began a CS taper at induction week 4 and continued the taper during maintenance; those who initiated CS during the study started the CS taper on symptom improvement. Endpoints included the proportion of patients without CS use (CS-free) at week 12 or for ≥ 90 days before week 52 who achieved clinical remission by stool frequency/abdominal pain score (SF/APS) or by CD Activity Index (CDAI), enhanced clinical response, decrease of ≥ 100 points in CDAI from baseline (CR-100), endoscopic remission, and endoscopic response at week 12 and week 52. CS daily dose (in prednisone-equivalent doses) was recorded.
Results: Of 1021 patients evaluated, the mean (SD) cumulative CS exposure over 12 weeks was 16.0 (19.0) mg daily of prednisone equivalent for UPA 45 mg and 24.0 (34.0) mg for PBO, and during maintenance, 13.7 (16.3) mg for UPA 15 mg, 13.5 (27.3) mg for UPA 30 mg, and 13.7 (9.9) mg for PBO. A significantly higher proportion of patients who received UPA vs PBO were CS-free and achieved clinical remission (by SF/APS and CDAI), enhanced clinical response, CR-100, endoscopic remission, and endoscopic response at week 12 (Fig 1A). At week 52, response rates across all assessed endpoints were generally maintained in patients who received UPA 15 or 30 mg, with significantly higher response rates in patients who received either UPA dose vs PBO (Fig 1B). Safety was as expected for UPA or CS.1
Discussion: During induction, higher proportions of patients treated with UPA were CS-free and experienced greater clinical and endoscopic improvements vs those receiving PBO. CS‑free response rates were generally sustained through week 52, suggesting that UPA may have an enduring steroid-sparing effect.
1. Loftus EV Jr, et al. N Engl J Med. 2023;388:1966–1980.

Figure: Figure 1. Proportion of Patients Who Were CS-Free and Achieved Clinical and Endoscopic Endpoints at Induction Week 12 (A) and Maintenance Week 52 (B) Among All Patients
APS, abdominal pain score; CDAI, Crohn’s Disease Activity Index; CR-100, clinical response 100; CS, corticosteroids; NRI-C, non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; PBO, placebo; SES-CD, Simplified Endoscopic Score for Crohn’s Disease; SF, stool frequency; UPA, upadacitinib.
Only patients with clinical response (≥ 30% decrease in average daily very soft or liquid SF and/or ≥ 30% decrease in average daily APS and both not worse than baseline) in the induction studies were eligible to enter the maintenance study.
For all assessments, NRI-C was used for incorporating multiple imputation to handle missing data due to COVID-19.
Data are percent of patients (95% CI).
Values above bars are percent of patients and n/N.
CS-free in the induction studies: Patients not taking CS at week 12.
CS-free in the maintenance study: Patients not taking CS within 90 days before week 52.
SF/APS clinical remission: Average daily very soft or liquid SF ≤ 2.8 and average daily APS ≤ 1.0 and both not greater than baseline.
CDAI clinical remission: CDAI < 150.
Enhanced clinical response: ≥ 60% decrease in average daily very soft or liquid SF and/or ≥ 35% decrease in average daily APS and both not greater than baseline, or clinical remission.
CR-100: Decrease of ≥ 100 points in CDAI from baseline.
Endoscopic remission: SES-CD ≤ 4 and ≥ 2-point reduction from baseline and no subscore > 1 in any individual variable.
Endoscopic response: Decrease in SES-CD > 50% from baseline of the induction period (or at least a 2-point reduction from baseline for patients with an SES-CD of 4 at baseline).
***P < .001 vs PBO.
APS, abdominal pain score; CDAI, Crohn’s Disease Activity Index; CR-100, clinical response 100; CS, corticosteroids; NRI-C, non-responder imputation incorporating multiple imputation to handle missing data due to COVID-19; PBO, placebo; SES-CD, Simplified Endoscopic Score for Crohn’s Disease; SF, stool frequency; UPA, upadacitinib.
Only patients with clinical response (≥ 30% decrease in average daily very soft or liquid SF and/or ≥ 30% decrease in average daily APS and both not worse than baseline) in the induction studies were eligible to enter the maintenance study.
For all assessments, NRI-C was used for incorporating multiple imputation to handle missing data due to COVID-19.
Data are percent of patients (95% CI).
Values above bars are percent of patients and n/N.
CS-free in the induction studies: Patients not taking CS at week 12.
CS-free in the maintenance study: Patients not taking CS within 90 days before week 52.
SF/APS clinical remission: Average daily very soft or liquid SF ≤ 2.8 and average daily APS ≤ 1.0 and both not greater than baseline.
CDAI clinical remission: CDAI < 150.
Enhanced clinical response: ≥ 60% decrease in average daily very soft or liquid SF and/or ≥ 35% decrease in average daily APS and both not greater than baseline, or clinical remission.
CR-100: Decrease of ≥ 100 points in CDAI from baseline.
Endoscopic remission: SES-CD ≤ 4 and ≥ 2-point reduction from baseline and no subscore > 1 in any individual variable.
Endoscopic response: Decrease in SES-CD > 50% from baseline of the induction period (or at least a 2-point reduction from baseline for patients with an SES-CD of 4 at baseline).
***P < .001 vs PBO.
Disclosures:
Marla Dubinsky: AbbVie – received consulting fees and/or acted as an advisor. Abivax – received consulting fees and/or acted as an advisor. AstraZeneca – received consulting fees and/or acted as an advisor. Bristol-Myers Squibb – received consulting fees and/or acted as an advisor. Eli Lilly – received consulting fees and/or acted as an advisor. Gilead – received consulting fees and/or acted as an advisor. Janssen – received consulting fees and/or acted as an advisor. Merck – received consulting fees and/or acted as an advisor. Pfizer – received consulting fees and/or acted as an advisor. Prometheus Biosciences – received consulting fees and/or acted as an advisor. Prometheus Labs – received consulting fees and/or acted as an advisor. Takeda – received consulting fees and/or acted as an advisor.
Geert D'Haens: AbbVie – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Cellitrion – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. GlaxoSmithKline – Consultant, Speakers Bureau. Gossamerbio – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Lilly – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus Biosciences – Consultant, Speakers Bureau. Prometheus Laboratories – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillotts – Consultant, Speakers Bureau.
Olivier Dewit: AbbVie – Consultant, Speakers Bureau. Biogen – Consultant, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Merck – Consultant, Speakers Bureau. Mylan – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau.
Pascal Juillerat: AbbVie – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Arena – Employee, Speakers Bureau. Bristol Myers Squibb – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Lilly – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Pierre Fabre – Consultant, Speakers Bureau. Roche – Consultant, Speakers Bureau. Sandoz – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillotts – Consultant, Speakers Bureau. UCB – Consultant, Speakers Bureau. Vifor – Grant/Research Support.
Remo Panaccione: Abbivax – Consultant. Abbott – Consultant. AbbVie – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena – Consultant. AstraZeneca – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Celltrion – Consultant. Cosmos Technology – Consultant. Eisai – Consultant. Elan – Consultant. Eli Lilly – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. JAMP Bio – Consultant. Janssen – Consultant. Merck – Consultant. Mylan – Consultant. Novartis – Consultant. Oppilan Pharma – Consultant. Organon – Consultant. Pandion Pharma – Consultant. Pendopharm – Consultant. Pfizer Inc – Consultant. Progenity – Consultant. Prometheus – Consultant. Protagonist Therapeutics – Consultant. Roche – Consultant. Sandoz – Consultant. Satisfai Health – Consultant. Shire – Consultant. Sublimity Therapeutics – Consultant. Takeda Pharmaceuticals – Consultant. Theravance Biopharma – Consultant. Trellus – Consultant. UCB – Consultant. Ventyx – Consultant. Viatris – Consultant.
Toshimitsu Fujii: AbbVie – Grant/Research Support, Speakers Bureau. Ajinomoto Pharma – Speakers Bureau. Alfresa – Grant/Research Support. Boehringer Ingelheim – Grant/Research Support, Speakers Bureau. Bristol-Myers Squibb – Grant/Research Support, Speakers Bureau. Celgene – Grant/Research Support. Daiichi Sankyo – Speakers Bureau. EA Pharma – Grant/Research Support, Speakers Bureau. Eisai – Grant/Research Support, Speakers Bureau. Gilead Sciences – Grant/Research Support. Janssen – Grant/Research Support, Speakers Bureau. Kissei – Grant/Research Support, Speakers Bureau. Kyorin – Speakers Bureau. Kyowa Hakko Kirin – Speakers Bureau. Lilly – Grant/Research Support. Mebix – Grant/Research Support. Mitsubishi-Tanabe – Speakers Bureau. Mochida – Speakers Bureau. Nichiiko – Speakers Bureau. Nippon Kayaku – Speakers Bureau. Pfizer – Speakers Bureau. Sanofi – Grant/Research Support. Taiho – Speakers Bureau. Takeda – Grant/Research Support, Speakers Bureau. Zeria Pharma – Speakers Bureau.
Ana Lacerda: AbbVie – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Elena Dubcenco: AbbVie – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Samuel Anyanwu: AbbVie – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Chirag Doshi: AbbVie – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Jianzhong Liu: AbbVie – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Andrew Garrison: AbbVie – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Edward Loftus: AbbVie – Consultant, Grant/Research Support. Alvotech – Consultant. Amgen – Consultant. Arena – Consultant. Avalo Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support. Celgene – Consultant, Grant/Research Support. Celltrion – Consultant. Exact Sciences – Stock-publicly held company(excluding mutual/index funds). Fresenius Kabi – Consultant. Genentech – Consultant, Grant/Research Support. Gilead – Consultant, Grant/Research Support. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant, Grant/Research Support. Iota Biosciences – Consultant. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support. KSL Diagnostics – Consultant. Lilly – Consultant. Morphic – Consultant. Ono Pharma – Consultant. Pfizer – Consultant, Grant/Research Support. Protagonist – Consultant. Receptos – Grant/Research Support. Robarts Clinical Trials – Grant/Research Support. Scipher Medicine – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support. Theravance – Grant/Research Support. UCB – Consultant, Grant/Research Support.
Marla C.. Dubinsky, MD1, Geert D'Haens, MD, PhD2, Olivier Dewit, MD, PhD3, Pascal Juillerat, MD, MSc4, Remo Panaccione, MD5, Toshimitsu Fujii, MD, PhD6, Ana P.. Lacerda, MD, MSc7, Elena Dubcenco, MD, MS7, Samuel I.. Anyanwu, PhD, MS7, Chirag Doshi, PharmD7, Jianzhong Liu, MD, MS7, Andrew Garrison, MS7, Edward V.. Loftus, MD8. P3598 - Corticosteroid-Sparing Effect of Upadacitinib in Patients With Moderately-to-Severely Active Crohn’s Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
