Tuesday Poster Session
Category: IBD
P3637 - Tofacitinib in Ulcerative Colitis: The Relationship Between OCTAVE Induction Week 8 Symptomatic Remission and OCTAVE Sustain Week 52 Remission
Tuesday, October 24, 2023
10:30 AM - 4:00 PM PT
Location: Exhibit Hall
Has Audio
- CH
Christina Ha, MD
Mayo Clinic Arizona
Scottsdale, AZ
Presenting Author(s)
Pierre Desreumaux, 1, Filiz Akyuz, 2, Christina Ha, MD3, Amira Bouzidi, 4, Amel Tamzali, 4, Sean Gardiner, 5, Joseph Wu, PhD6, Jerome Paulissen, 5, Lucine Vuitton, 7, Miguel Regueiro, MD8
1Lille University and Hospital, INFINITE, U1286 Inserm, Lille, Haute-Normandie, France; 2Istanbul University, Istanbul, Istanbul, Turkey; 3Mayo Clinic Arizona, Scottsdale, AZ; 4Pfizer Inc., Paris, Ile-de-France, France; 5Pfizer Inc., New York, NY; 6Pfizer Inc., Cambridge, MA; 7University Hospital of Besançon, University Bourgogne-Franche-Comté, Besançon, Bourgogne, France; 8Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH
Introduction: Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis.
Methods: This post hoc analysis aimed to 1) identify baseline factors associated with symptomatic remission at Week 8 in patients who received tofacitinib 10 mg twice daily (BID) in OCTAVE Induction 1&2 (NCT01465763; NCT01458951),1 and 2) compare the efficacy of tofacitinib 5 and 10 mg BID after 52 weeks of maintenance treatment in OCTAVE Sustain (NCT01458574)1 in patients who achieved symptomatic remission at maintenance baseline (ie after 8 weeks of induction therapy) vs those who did not. Logistic regression analysis was performed for symptomatic remission as the dependent variable, with each induction baseline predictor as an independent covariate. The proportions of patients in remission, corticosteroid-free remission (CSFR), and sustained CSFR at Week 52 were summarized in those who were symptomatic remitters vs non-remitters at maintenance baseline.
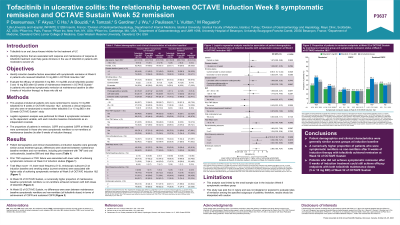
Results: Prior tumor necrosis factor inhibitor exposure or failure was associated with lower odds of achieving symptomatic remission at Week 8 of induction studies (odds ratio [OR] 0.44, 95% confidence interval [CI] 0.25, 0.77; OR 0.42, 95% CI 0.23, 0.75; respectively). Baseline total Mayo score (< 9 vs ≥ 9) and lower stool frequency (2 vs 3) and endoscopic subscore (2 vs 3) were associated with higher odds of achieving symptomatic remission at Week 8 of induction studies (OR 2.82, 95% CI 1.63, 4.88; OR 3.04, 95% CI 1.63, 5.65; OR 2.86, 95% CI 1.65, 4.97; respectively). A numerically higher proportion of maintenance baseline symptomatic remitters vs non-remitters achieved remission at Week 52 (47.1% vs 34.2%; all tofacitinib doses). No differences were seen between maintenance baseline symptomatic remitters and non-remitters at Week 52 (all tofacitinib doses) in terms of achievement of CSFR (26.9 vs 26.3%, respectively) and sustained CSFR (41.2% vs 41.0%, respectively; Table).
Discussion: A numerically higher proportion of patients who were symptomatic remitters vs non-remitters after 8 weeks of induction therapy with tofacitinib achieved remission at Week 52 of OCTAVE Sustain, but those who did not achieve symptomatic remission after 8 weeks of induction treatment could still achieve efficacy endpoints with both tofacitinib maintenance doses at Week 52.
Reference
1. Sandborn et al. N Engl J Med 2017;376:1723–36
Disclosures:
Pierre Desreumaux, 1, Filiz Akyuz, 2, Christina Ha, MD3, Amira Bouzidi, 4, Amel Tamzali, 4, Sean Gardiner, 5, Joseph Wu, PhD6, Jerome Paulissen, 5, Lucine Vuitton, 7, Miguel Regueiro, MD8. P3637 - Tofacitinib in Ulcerative Colitis: The Relationship Between OCTAVE Induction Week 8 Symptomatic Remission and OCTAVE Sustain Week 52 Remission, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Lille University and Hospital, INFINITE, U1286 Inserm, Lille, Haute-Normandie, France; 2Istanbul University, Istanbul, Istanbul, Turkey; 3Mayo Clinic Arizona, Scottsdale, AZ; 4Pfizer Inc., Paris, Ile-de-France, France; 5Pfizer Inc., New York, NY; 6Pfizer Inc., Cambridge, MA; 7University Hospital of Besançon, University Bourgogne-Franche-Comté, Besançon, Bourgogne, France; 8Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH
Introduction: Tofacitinib is an oral small molecule Janus kinase inhibitor for the treatment of ulcerative colitis.
Methods: This post hoc analysis aimed to 1) identify baseline factors associated with symptomatic remission at Week 8 in patients who received tofacitinib 10 mg twice daily (BID) in OCTAVE Induction 1&2 (NCT01465763; NCT01458951),1 and 2) compare the efficacy of tofacitinib 5 and 10 mg BID after 52 weeks of maintenance treatment in OCTAVE Sustain (NCT01458574)1 in patients who achieved symptomatic remission at maintenance baseline (ie after 8 weeks of induction therapy) vs those who did not. Logistic regression analysis was performed for symptomatic remission as the dependent variable, with each induction baseline predictor as an independent covariate. The proportions of patients in remission, corticosteroid-free remission (CSFR), and sustained CSFR at Week 52 were summarized in those who were symptomatic remitters vs non-remitters at maintenance baseline.
Results: Prior tumor necrosis factor inhibitor exposure or failure was associated with lower odds of achieving symptomatic remission at Week 8 of induction studies (odds ratio [OR] 0.44, 95% confidence interval [CI] 0.25, 0.77; OR 0.42, 95% CI 0.23, 0.75; respectively). Baseline total Mayo score (< 9 vs ≥ 9) and lower stool frequency (2 vs 3) and endoscopic subscore (2 vs 3) were associated with higher odds of achieving symptomatic remission at Week 8 of induction studies (OR 2.82, 95% CI 1.63, 4.88; OR 3.04, 95% CI 1.63, 5.65; OR 2.86, 95% CI 1.65, 4.97; respectively). A numerically higher proportion of maintenance baseline symptomatic remitters vs non-remitters achieved remission at Week 52 (47.1% vs 34.2%; all tofacitinib doses). No differences were seen between maintenance baseline symptomatic remitters and non-remitters at Week 52 (all tofacitinib doses) in terms of achievement of CSFR (26.9 vs 26.3%, respectively) and sustained CSFR (41.2% vs 41.0%, respectively; Table).
Discussion: A numerically higher proportion of patients who were symptomatic remitters vs non-remitters after 8 weeks of induction therapy with tofacitinib achieved remission at Week 52 of OCTAVE Sustain, but those who did not achieve symptomatic remission after 8 weeks of induction treatment could still achieve efficacy endpoints with both tofacitinib maintenance doses at Week 52.
Reference
1. Sandborn et al. N Engl J Med 2017;376:1723–36
| Outcome at Week 52 of OCTAVE Sustain | Maintenance treatment | ||
| Tofacitinib 5 mg BID | Tofacitinib 10 mg BID | All tofacitinib doses | |
| Remission, n/N (%) | |||
| Symptomatic remission status at Week 8 of OCTAVE Induction 1&2: | |||
| Yes[b] | 14/36 (38.9) | 18/32 (56.3) | 32/68 (47.1) |
| No | 41/134 (30.6) | 51/135 (37.8) | 92/269 (34.2) |
| CSFR, n/N (%)[c] | |||
| Symptomatic remission status at Week 8 of OCTAVE Induction 1&2: | |||
| Yes[b] | 4/16 (25.0) | 3/10 (30.0) | 7/26 (26.9) |
| No | 19/72 (26.4) | 16/61 (26.2) | 35/133 (26.3) |
| Sustained CSFR, n/N (%)[d] | |||
| Symptomatic remission status at Week 8 of OCTAVE Induction 1&2: | |||
| Yes[b] | 11/36 (30.6) | 17/32 (53.1) | 28/68 (41.2) |
| No | 9/21 (42.9) | 7/18 (38.9) | 16/39 (41.0) |
Disclosures:
Pierre Desreumaux: Abbott – Advisory Committee/Board Member, Consultant. AbbVie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Biocodex – Advisory Committee/Board Member, Consultant. BioFortis – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. BioKuris – Advisory Committee/Board Member, Consultant. Ferring – Advisory Committee/Board Member, Consultant. Fresenius – Advisory Committee/Board Member, Consultant. Intralytix – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. KitoZyme – Advisory Committee/Board Member, Consultant. Lesaffre – Advisory Committee/Board Member, Consultant. MSD – Advisory Committee/Board Member, Consultant. Nestlé – Advisory Committee/Board Member, Consultant. Nogra – Advisory Committee/Board Member, Consultant. Norgine – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. Roquette – Advisory Committee/Board Member, Consultant. Sandox – Advisory Committee/Board Member, Consultant. Shire – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Tillots – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant.
Filiz Akyuz: Celltrion – Speakers Bureau. Ferring – Speakers Bureau. Pfizer Inc – Speakers Bureau.
Christina Ha: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support. Bristol-Myers Squibb – Advisory Committee/Board Member, Consultant. Helmsley Charitable Trust, – Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Lily – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant.
Amira Bouzidi: Pfizer Inc – Employee, Personal Fees.
Amel Tamzali: Pfizer Inc – Employee, Personal Fees.
Sean Gardiner: Pfizer Inc – Employee, Shareholder.
Joseph Wu: Pfizer Inc – Employee, Stock Options.
Jerome Paulissen: Eli Lilly – contractor. Pfizer Inc – Paid Contractor. Syneos Health – Employee.
Lucine Vuitton: AbbVie – Fees. Amgen – Fees. Biogen – Fees. Celltrion – Fees. Eli Lilly – Fees. Ferring – Fees. Galapagos – Fees. Janssen – Fees. MSD – Fees. Pfizer Inc – Fees. Takeda – Fees. Viatris – Fees.
Miguel Regueiro: AbbVie – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Alfasigma – Advisory Committee/Board Member, Consultant. Allergan – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Celgene – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Eli Lilly – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Gilead Sciences – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Janssen – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Miraca Labs – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Prometheus – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Seres – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Target RWE – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Wolters Kluwer Health – Royalties.
Pierre Desreumaux, 1, Filiz Akyuz, 2, Christina Ha, MD3, Amira Bouzidi, 4, Amel Tamzali, 4, Sean Gardiner, 5, Joseph Wu, PhD6, Jerome Paulissen, 5, Lucine Vuitton, 7, Miguel Regueiro, MD8. P3637 - Tofacitinib in Ulcerative Colitis: The Relationship Between OCTAVE Induction Week 8 Symptomatic Remission and OCTAVE Sustain Week 52 Remission, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
