Monday Poster Session
Category: Biliary/Pancreas
P1425 - Spontaneous Migration of Pancreatic Stents Placed for Post-ERCP Pancreatitis Prophylaxis: Rates and Time to Migration
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Mark A. Gromski, MD
Indiana University School of Medicine
Indianapolis, IN
Presenting Author(s)
Mark A. Gromski, MD1, Nicholas D. Dey, MS, PhD2, Binhan Wang, MS2, Jennifer Gatz, PhD3, Stephen O'Brien, BS3, Ryan C. Cutshall, MPH3, Krishna C. Vemulapalli, MBBS, MPH2
1Indiana University School of Medicine, Indianapolis, IN; 2Cook Research Incorporated, West Lafayette, IN; 3Regenstrief Institute, Indianapolis, IN
Introduction: Multiple society guidelines recommend placing pancreatic stents for post-ERCP (endoscopic retrograde cholangiopancreatography) pancreatitis prophylaxis in select cases. There is limited data on spontaneous migration and time to migration of these devices.
Methods: Data on consecutive patients where a pancreatic stent (Cook Medical, Bloomington, IN) was used during an ERCP procedure between September 2019 and December 2019 were collected from an Indiana state-wide medical records database. Data were collected as part of post-market clinical follow up efforts with the primary aims of establishing continuing safety and effectiveness for the drainage of obstructed pancreatic ducts in a real-world setting and to identify potential systematic off-label use. Data elements included indication for procedure, duct status during follow-up, time to migration or removal, clinical success, and any adverse events.
Results: Pancreatic stent placement was attempted in 128 patients (mean age 57.3 years; 59.4% (76/128) female; 87.5% (112/118) were White) (Table 1).
Successful stent placement was achieved among 98.4% (126/128) of patients. Clinical success was achieved among 92.9% (117/126) of patients. Thirty-four (26.6%) patients reported at least 1 adverse event with the most frequent being pain or discomfort associated with the procedure (20.3%, 26/128), most classified as mild. Post-ERCP pancreatitis related to the device or procedure was identified in 3.9% (5/128) of patients.
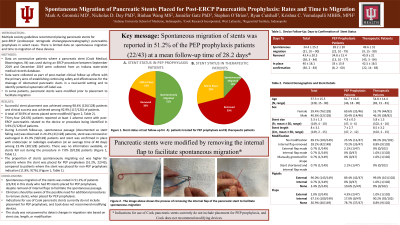
Spontaneous migration was observed among 51.2% (22/43) of patients with PEP prophylaxis as the indication vs. 11.8% (9/76) of patients with other indications. The mean days to confirmation of the stent spontaneously migrating out in the PEP prophylaxis patients was 28.2 days (range: 10 days-79 days) (Table 1).
Discussion: ESGE guidelines recommend pancreatic stents to be in place no longer than 10 days when placed for PEP prophylaxis. Spontaneous migration of the stents was noted only half the time in PEP prophylaxis patients (51.2%, 22/43) in this study. This is despite removal of internal flaps (Figure 1) to facilitate the spontaneous passage. Clinicians should be aware of the possibility for additional procedures to remove stents, when placed for PEP prophylaxis. Indications for use of Cook pancreatic stents currently do not include placement for PEP prophylaxis, and Cook does not recommend modifying devices. The study was not powered to detect changes in migration rate based on stent size, length, or modification.

Disclosures:
Mark A. Gromski, MD1, Nicholas D. Dey, MS, PhD2, Binhan Wang, MS2, Jennifer Gatz, PhD3, Stephen O'Brien, BS3, Ryan C. Cutshall, MPH3, Krishna C. Vemulapalli, MBBS, MPH2. P1425 - Spontaneous Migration of Pancreatic Stents Placed for Post-ERCP Pancreatitis Prophylaxis: Rates and Time to Migration, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Indiana University School of Medicine, Indianapolis, IN; 2Cook Research Incorporated, West Lafayette, IN; 3Regenstrief Institute, Indianapolis, IN
Introduction: Multiple society guidelines recommend placing pancreatic stents for post-ERCP (endoscopic retrograde cholangiopancreatography) pancreatitis prophylaxis in select cases. There is limited data on spontaneous migration and time to migration of these devices.
Methods: Data on consecutive patients where a pancreatic stent (Cook Medical, Bloomington, IN) was used during an ERCP procedure between September 2019 and December 2019 were collected from an Indiana state-wide medical records database. Data were collected as part of post-market clinical follow up efforts with the primary aims of establishing continuing safety and effectiveness for the drainage of obstructed pancreatic ducts in a real-world setting and to identify potential systematic off-label use. Data elements included indication for procedure, duct status during follow-up, time to migration or removal, clinical success, and any adverse events.
Results: Pancreatic stent placement was attempted in 128 patients (mean age 57.3 years; 59.4% (76/128) female; 87.5% (112/118) were White) (Table 1).
Successful stent placement was achieved among 98.4% (126/128) of patients. Clinical success was achieved among 92.9% (117/126) of patients. Thirty-four (26.6%) patients reported at least 1 adverse event with the most frequent being pain or discomfort associated with the procedure (20.3%, 26/128), most classified as mild. Post-ERCP pancreatitis related to the device or procedure was identified in 3.9% (5/128) of patients.
Spontaneous migration was observed among 51.2% (22/43) of patients with PEP prophylaxis as the indication vs. 11.8% (9/76) of patients with other indications. The mean days to confirmation of the stent spontaneously migrating out in the PEP prophylaxis patients was 28.2 days (range: 10 days-79 days) (Table 1).
Discussion: ESGE guidelines recommend pancreatic stents to be in place no longer than 10 days when placed for PEP prophylaxis. Spontaneous migration of the stents was noted only half the time in PEP prophylaxis patients (51.2%, 22/43) in this study. This is despite removal of internal flaps (Figure 1) to facilitate the spontaneous passage. Clinicians should be aware of the possibility for additional procedures to remove stents, when placed for PEP prophylaxis. Indications for use of Cook pancreatic stents currently do not include placement for PEP prophylaxis, and Cook does not recommend modifying devices. The study was not powered to detect changes in migration rate based on stent size, length, or modification.

Figure: a,b Stent prior to modification with a flap; c,d Flap being cut; e,f Stent post modification without flap
Disclosures:
Mark Gromski: Ambu – Consultant. Boston Scientific – Consultant. Cook Medical – Grant/Research Support.
Nicholas Dey: Cook Research Incorporated – Employee.
Binhan Wang: Cook Research Incorporated – Employee.
Jennifer Gatz: Cook – Grant/Research Support. Janssen – Grant/Research Support.
Stephen O'Brien indicated no relevant financial relationships.
Ryan Cutshall indicated no relevant financial relationships.
Krishna Vemulapalli: Cook Research Incorporated – Employee.
Mark A. Gromski, MD1, Nicholas D. Dey, MS, PhD2, Binhan Wang, MS2, Jennifer Gatz, PhD3, Stephen O'Brien, BS3, Ryan C. Cutshall, MPH3, Krishna C. Vemulapalli, MBBS, MPH2. P1425 - Spontaneous Migration of Pancreatic Stents Placed for Post-ERCP Pancreatitis Prophylaxis: Rates and Time to Migration, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
