Monday Poster Session
Category: Biliary/Pancreas
P1535 - Choledocholithiasis Mimicking Testosterone-Induced Hepatitis in a Transgender Male
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Alexander M. Carlson, DO
University at Buffalo
Buffalo, NY
Presenting Author(s)
Alexander M. Carlson, DO1, Clive J. Miranda, DO, MS1, Navpreet K. Rana, DO, MS2, Thomas C. Mahl, MD1, Charles G. Carlson, BS3
1University at Buffalo, Buffalo, NY; 2University at Buffalo, Orchard Park, NY; 3SUNY Upstate Medical University, Syracuse, NY
Introduction: Many patients with gender dysphoria undergo hormone therapy as part of the transition process to facilitate congruence between their sex and gender identity. There is a mild concern regarding the use of testosterone therapy and its risk of hepatotoxicity with certain isoforms. Here we describe a transgender male who recently started gender transition therapy who was found to have choledocholithiasis, initially thought to be testosterone-induced hepatoxicity.
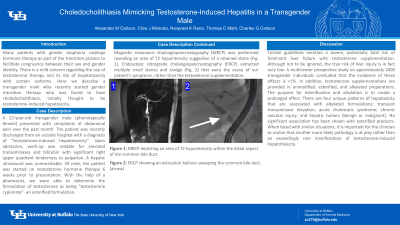
Case Description/Methods: A 22-year-old transgender male (phenotypically female) presented with complaints of abdominal pain over the past month. The patient was recently discharged from an outside hospital with a diagnosis of “testosterone-induced hepatotoxicity”. Upon admission, work-up was notable for elevated transaminases and bilirubin with significant right upper quadrant tenderness to palpation. A hepatic ultrasound was unremarkable. Of note, the patient was started on testosterone hormone therapy 6 weeks prior to presentation. With the help of a pharmacist, we were able to determine the formulation of testosterone as being "testosterone cypionate”- an esterified formulation. Magnetic resonance cholangiopancreatography (MRCP) was performed revealing an area of T2 hypointensity suggestive of a retained stone (Fig. 1). Endoscopic retrograde cholangiopancreatography (ERCP) extracted multiple small stones and sludge (Fig. 2) that were the cause of our patient’s symptoms, rather than the testosterone supplementation.
Discussion: Certain guidelines mention a severe, potentially fatal risk of fulminant liver failure with testosterone supplementation. Although not to be ignored, the true risk of liver injury is in fact very low. A multicenter prospective study on approximately 2000 transgender individuals concluded that the incidence of these effects is < 1%. In addition, testosterone supplementations are provided in unmodified, esterified, and alkylated preparations. The purpose for esterification and alkylation is to create a prolonged effect. There are four unique patterns of hepatoxicity that are associated with alkylated formulations: transient transaminase elevation; acute cholestatic syndrome; chronic vascular injury; and hepatic tumors (benign or malignant). No significant association has been shown with esterified products. When faced with similar situations, it is important for the clinician to realize that another more likely pathology is at play rather than an exceedingly rare manifestation of testosterone-induced hepatotoxicity.

Disclosures:
Alexander M. Carlson, DO1, Clive J. Miranda, DO, MS1, Navpreet K. Rana, DO, MS2, Thomas C. Mahl, MD1, Charles G. Carlson, BS3. P1535 - Choledocholithiasis Mimicking Testosterone-Induced Hepatitis in a Transgender Male, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University at Buffalo, Buffalo, NY; 2University at Buffalo, Orchard Park, NY; 3SUNY Upstate Medical University, Syracuse, NY
Introduction: Many patients with gender dysphoria undergo hormone therapy as part of the transition process to facilitate congruence between their sex and gender identity. There is a mild concern regarding the use of testosterone therapy and its risk of hepatotoxicity with certain isoforms. Here we describe a transgender male who recently started gender transition therapy who was found to have choledocholithiasis, initially thought to be testosterone-induced hepatoxicity.
Case Description/Methods: A 22-year-old transgender male (phenotypically female) presented with complaints of abdominal pain over the past month. The patient was recently discharged from an outside hospital with a diagnosis of “testosterone-induced hepatotoxicity”. Upon admission, work-up was notable for elevated transaminases and bilirubin with significant right upper quadrant tenderness to palpation. A hepatic ultrasound was unremarkable. Of note, the patient was started on testosterone hormone therapy 6 weeks prior to presentation. With the help of a pharmacist, we were able to determine the formulation of testosterone as being "testosterone cypionate”- an esterified formulation. Magnetic resonance cholangiopancreatography (MRCP) was performed revealing an area of T2 hypointensity suggestive of a retained stone (Fig. 1). Endoscopic retrograde cholangiopancreatography (ERCP) extracted multiple small stones and sludge (Fig. 2) that were the cause of our patient’s symptoms, rather than the testosterone supplementation.
Discussion: Certain guidelines mention a severe, potentially fatal risk of fulminant liver failure with testosterone supplementation. Although not to be ignored, the true risk of liver injury is in fact very low. A multicenter prospective study on approximately 2000 transgender individuals concluded that the incidence of these effects is < 1%. In addition, testosterone supplementations are provided in unmodified, esterified, and alkylated preparations. The purpose for esterification and alkylation is to create a prolonged effect. There are four unique patterns of hepatoxicity that are associated with alkylated formulations: transient transaminase elevation; acute cholestatic syndrome; chronic vascular injury; and hepatic tumors (benign or malignant). No significant association has been shown with esterified products. When faced with similar situations, it is important for the clinician to realize that another more likely pathology is at play rather than an exceedingly rare manifestation of testosterone-induced hepatotoxicity.

Figure: Figure 1: MRCP depicting an area of T2 hypointensity within the distal aspect of the common bile duct.
Figure 2: ERCP showing an extraction balloon sweeping the common bile duct. (arrow)
Figure 2: ERCP showing an extraction balloon sweeping the common bile duct. (arrow)
Disclosures:
Alexander Carlson indicated no relevant financial relationships.
Clive Miranda indicated no relevant financial relationships.
Navpreet Rana indicated no relevant financial relationships.
Thomas Mahl indicated no relevant financial relationships.
Charles Carlson indicated no relevant financial relationships.
Alexander M. Carlson, DO1, Clive J. Miranda, DO, MS1, Navpreet K. Rana, DO, MS2, Thomas C. Mahl, MD1, Charles G. Carlson, BS3. P1535 - Choledocholithiasis Mimicking Testosterone-Induced Hepatitis in a Transgender Male, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
