Monday Poster Session
Category: Colon
P1582 - Tumor Microenvironment Analysis in Metastatic Sites of Colorectal Cancer
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- IC
I-Ling Chiang, MD
Vanderbilt University Medical Center
Nashville, Tennessee
Presenting Author(s)
Award: Presidential Poster Award
I-Ling Chiang, MD1, Michael D. Iglesia, MD, PhD2, Reyka G. Jayasinghe, PhD2, Jingxian Liu, 2, Andrew Houston, 2, John M. Herndon, 3, Jacqueline Mudd, 2, Ryan C. Fields, MD2, Li Ding, PhD2
1Vanderbilt University Medical Center, Nashville, TN; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3Washington University in St. Louis, St. Louis, MO
Introduction: Colorectal cancer is the third most common cancer globally and metastatic colorectal cancer in most cases is still considered incurable. Thus, there is a need to expand our understanding of metastatic colorectal cancer. There is growing evidence that tissue-specific microenvironmental factors may shape the pattern of metastatic spread. In this study, we analyzed the immune cells of the tumor microenvironment, focusing on the myeloid lineage. We hypothesized that the distribution of myeloid cells would be dependent on the site of metastases.
Methods: Samples from patients with metastatic colorectal cancer were collected from surgically resected specimens. Cells were analyzed by single-nucleus RNA sequencing. Data was processed via in-house pipeline and cells belonging to the myeloid lineage were separated out for detailed analysis. Quantification and visualization of the data was performed with Seurat.
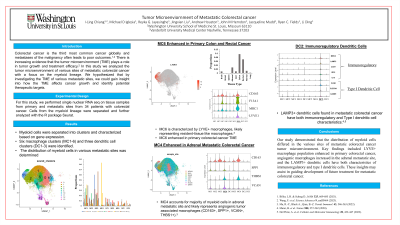
Results: Myeloid cells from n=37 patients were isolated for analysis of the primary and metastatic tumor microenvironment. Cell types were delineated by performing differential gene expression. Three dendritic (DC1, DC2, DC3) and six macrophage (M1, M2, M3, M4, M5, M6) clusters were identified and verified by expression of published cell type specific gene signatures (Figure 1A). Overall, the macrophages isolated express features of angiogenic tumor associated macrophage/TAM (CD163, SPP1, VCAN, THBS1), lipid associated TAM (CD163, F13A1, APOE, MRC1), and resident-like TAM (LYVE1). By calculating the distribution of myeloid cells throughout the 9 cell types, we demonstrate differences suggesting alterations of the tumor microenvironment as the cancer develops in different organ sites (Figure 1B). Most saliently, M6 (F13A1+, MRC1+, LYVE+) was enriched in primary colorectal cancer myeloid cells (colon: 9.7% and rectum: 7.1%), M4 (THBS+, VAN+) was enriched in adrenal myeloid cells (83.5%), and DC-2 (LAMP3+,BIRC3+,CCR7+)/DC-3 (TCF4+,IRF4+,PLAC8+) enhanced in lung/breast cancer myeloid cells.
Discussion: There is evidence suggesting that the tumor microenvironment differs in the various organs. This information will be vital in improving and designing therapies for metastatic cancer patients.

Disclosures:
I-Ling Chiang, MD1, Michael D. Iglesia, MD, PhD2, Reyka G. Jayasinghe, PhD2, Jingxian Liu, 2, Andrew Houston, 2, John M. Herndon, 3, Jacqueline Mudd, 2, Ryan C. Fields, MD2, Li Ding, PhD2. P1582 - Tumor Microenvironment Analysis in Metastatic Sites of Colorectal Cancer, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
I-Ling Chiang, MD1, Michael D. Iglesia, MD, PhD2, Reyka G. Jayasinghe, PhD2, Jingxian Liu, 2, Andrew Houston, 2, John M. Herndon, 3, Jacqueline Mudd, 2, Ryan C. Fields, MD2, Li Ding, PhD2
1Vanderbilt University Medical Center, Nashville, TN; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3Washington University in St. Louis, St. Louis, MO
Introduction: Colorectal cancer is the third most common cancer globally and metastatic colorectal cancer in most cases is still considered incurable. Thus, there is a need to expand our understanding of metastatic colorectal cancer. There is growing evidence that tissue-specific microenvironmental factors may shape the pattern of metastatic spread. In this study, we analyzed the immune cells of the tumor microenvironment, focusing on the myeloid lineage. We hypothesized that the distribution of myeloid cells would be dependent on the site of metastases.
Methods: Samples from patients with metastatic colorectal cancer were collected from surgically resected specimens. Cells were analyzed by single-nucleus RNA sequencing. Data was processed via in-house pipeline and cells belonging to the myeloid lineage were separated out for detailed analysis. Quantification and visualization of the data was performed with Seurat.
Results: Myeloid cells from n=37 patients were isolated for analysis of the primary and metastatic tumor microenvironment. Cell types were delineated by performing differential gene expression. Three dendritic (DC1, DC2, DC3) and six macrophage (M1, M2, M3, M4, M5, M6) clusters were identified and verified by expression of published cell type specific gene signatures (Figure 1A). Overall, the macrophages isolated express features of angiogenic tumor associated macrophage/TAM (CD163, SPP1, VCAN, THBS1), lipid associated TAM (CD163, F13A1, APOE, MRC1), and resident-like TAM (LYVE1). By calculating the distribution of myeloid cells throughout the 9 cell types, we demonstrate differences suggesting alterations of the tumor microenvironment as the cancer develops in different organ sites (Figure 1B). Most saliently, M6 (F13A1+, MRC1+, LYVE+) was enriched in primary colorectal cancer myeloid cells (colon: 9.7% and rectum: 7.1%), M4 (THBS+, VAN+) was enriched in adrenal myeloid cells (83.5%), and DC-2 (LAMP3+,BIRC3+,CCR7+)/DC-3 (TCF4+,IRF4+,PLAC8+) enhanced in lung/breast cancer myeloid cells.
Discussion: There is evidence suggesting that the tumor microenvironment differs in the various organs. This information will be vital in improving and designing therapies for metastatic cancer patients.

Figure: Figure 1. (A) Dot plot demonstrating signature gene expression of the various cell types. (B) Plot showing distribution of myeloid cells among the 9 identified cell types.
Disclosures:
I-Ling Chiang indicated no relevant financial relationships.
Michael Iglesia indicated no relevant financial relationships.
Reyka Jayasinghe indicated no relevant financial relationships.
Jingxian Liu indicated no relevant financial relationships.
Andrew Houston indicated no relevant financial relationships.
John Herndon indicated no relevant financial relationships.
Jacqueline Mudd indicated no relevant financial relationships.
Ryan Fields indicated no relevant financial relationships.
Li Ding indicated no relevant financial relationships.
I-Ling Chiang, MD1, Michael D. Iglesia, MD, PhD2, Reyka G. Jayasinghe, PhD2, Jingxian Liu, 2, Andrew Houston, 2, John M. Herndon, 3, Jacqueline Mudd, 2, Ryan C. Fields, MD2, Li Ding, PhD2. P1582 - Tumor Microenvironment Analysis in Metastatic Sites of Colorectal Cancer, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

