Monday Poster Session
Category: Colon
P1585 - First-Line Treatment of Fecal Microbiota Transplantation for Immune-Mediated Colitis
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- KV
Krishnavathana Varatharajalu, MD
University of Texas MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Yinghong Wang, MD1, Krishnavathana Varatharajalu, MD1, Malek Shatila, MD1, Anusha Thomas, MD1, Mathew Campbell, MD1, Pavlos Msaouel, MD1, Craig Kovitz, MD1, Herbert DuPont, MD2
1University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas School of Public Health, Houston, TX
Introduction: The management of moderate to severe immune-mediated colitis (IMC) includes immunosuppression with steroids and/or biologic agents. Long-term immunosuppression increases the risk for infections and steroid side-effects. Fecal microbiota transplantation (FMT) is increasingly used for the treatment of refractory IMC but has not been studied in the front-line setting. We activated an ongoing clinical trial to test the hypothesis that front-line FMT can alleviate IMC symptoms while reducing unnecessary exposure to steroids and their complications.
Methods: We will report interim data from a prospective clinical trial exploring the efficacy and safety of FMT as a first-line treatment for IMC. To be included, patients had to 1) have symptoms of immune-mediated diarrhea or colitis grade ≥ 2 within 45 days of FMT and 2) not have received any immunosuppressive treatment for IMC or any other indication around the time of FMT.
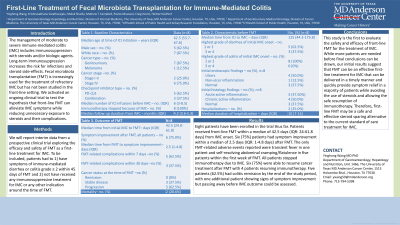
Results: Eight patients have been enrolled in the trial thus far. Patients received front-line FMT within a median of 42.5 days (IQR: 24-61.8 days) from IMC onset. Six (75%) patients had symptom improvement within a median of 2.5 days (IQR: 1-4.8 days) after FMT. The only FMT-related adverse events reported were transient fever in one patient and self-resolving abdominal cramping/flatulence in five patients within the first week of FMT. All patients stopped immunotherapy due to IMC. Six (75%) were able to resume cancer treatment after FMT with 4 patients resuming immunothreapy. Five patients (62.5%) had colitis remission by the end of the study period, with one additional patient showing signs of symptom improvement but passing away before IMC outcome could be assessed.
Discussion: This study is the first to evaluate the safety and efficacy of front-line FMT for the treatment of IMC. While more patients are needed before final conclusions can be drawn, our initial results suggest that FMT can be an effective first-line treatment for IMC that can be delivered in a timely manner and quickly provide symptom relief in a majority of patients while avoiding the use of steroids and allowing the safe resumption of immunotherapy. Therefore, first-line FMT may be a safe and effective steroid sparing alternative to the current standard of care treatment for IMC.
Disclosures:
Yinghong Wang, MD1, Krishnavathana Varatharajalu, MD1, Malek Shatila, MD1, Anusha Thomas, MD1, Mathew Campbell, MD1, Pavlos Msaouel, MD1, Craig Kovitz, MD1, Herbert DuPont, MD2. P1585 - First-Line Treatment of Fecal Microbiota Transplantation for Immune-Mediated Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas School of Public Health, Houston, TX
Introduction: The management of moderate to severe immune-mediated colitis (IMC) includes immunosuppression with steroids and/or biologic agents. Long-term immunosuppression increases the risk for infections and steroid side-effects. Fecal microbiota transplantation (FMT) is increasingly used for the treatment of refractory IMC but has not been studied in the front-line setting. We activated an ongoing clinical trial to test the hypothesis that front-line FMT can alleviate IMC symptoms while reducing unnecessary exposure to steroids and their complications.
Methods: We will report interim data from a prospective clinical trial exploring the efficacy and safety of FMT as a first-line treatment for IMC. To be included, patients had to 1) have symptoms of immune-mediated diarrhea or colitis grade ≥ 2 within 45 days of FMT and 2) not have received any immunosuppressive treatment for IMC or any other indication around the time of FMT.
Results: Eight patients have been enrolled in the trial thus far. Patients received front-line FMT within a median of 42.5 days (IQR: 24-61.8 days) from IMC onset. Six (75%) patients had symptom improvement within a median of 2.5 days (IQR: 1-4.8 days) after FMT. The only FMT-related adverse events reported were transient fever in one patient and self-resolving abdominal cramping/flatulence in five patients within the first week of FMT. All patients stopped immunotherapy due to IMC. Six (75%) were able to resume cancer treatment after FMT with 4 patients resuming immunothreapy. Five patients (62.5%) had colitis remission by the end of the study period, with one additional patient showing signs of symptom improvement but passing away before IMC outcome could be assessed.
Discussion: This study is the first to evaluate the safety and efficacy of front-line FMT for the treatment of IMC. While more patients are needed before final conclusions can be drawn, our initial results suggest that FMT can be an effective first-line treatment for IMC that can be delivered in a timely manner and quickly provide symptom relief in a majority of patients while avoiding the use of steroids and allowing the safe resumption of immunotherapy. Therefore, first-line FMT may be a safe and effective steroid sparing alternative to the current standard of care treatment for IMC.
Disclosures:
Yinghong Wang: ilyapharma – Consultant. IOTA – Consultant. Janssen – Consultant. MabQuest – Advisory Committee/Board Member. Sorriso – Consultant. Tillotts – Consultant.
Krishnavathana Varatharajalu indicated no relevant financial relationships.
Malek Shatila indicated no relevant financial relationships.
Anusha Thomas indicated no relevant financial relationships.
Mathew Campbell: Apricity Health – Grant/Research Support. Aravive – Grant/Research Support. Aveo – Advisory Committee/Board Member, Grant/Research Support. Eisai – Advisory Committee/Board Member. Exelixis – Advisory Committee/Board Member, Consultant, Grant/Research Support. Janssen – Grant/Research Support. Pfizer – Grant/Research Support. Seagen – Advisory Committee/Board Member, Grant/Research Support.
Pavlos Msaouel: BMS – Grant/Research Support. Mirati Therapeutics – Advisory board. Takeda – Grant/Research Support.
Craig Kovitz indicated no relevant financial relationships.
Herbert DuPont indicated no relevant financial relationships.
Yinghong Wang, MD1, Krishnavathana Varatharajalu, MD1, Malek Shatila, MD1, Anusha Thomas, MD1, Mathew Campbell, MD1, Pavlos Msaouel, MD1, Craig Kovitz, MD1, Herbert DuPont, MD2. P1585 - First-Line Treatment of Fecal Microbiota Transplantation for Immune-Mediated Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
