Monday Poster Session
Category: Colon
P1603 - The Interplay Between Immunocompromise and Immunotherapy Affects the Risk of Inflammatory Adverse Events
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
- MS
Malek Shatila, MD
University of Texas MD Anderson Cancer Center
Houston, Texas
Presenting Author(s)
Malek Shatila, MD1, Antonio Pizuorno Machado, MD2, Jay S. Shah, MD3, Sidra Naz, MD, MPH1, Nicholas Short, MD1, Anusha Thomas, MD1, Hao Chi Zhang, MD1, Yinghong Wang, MD1
1University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3Baylor College of Medicine, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) are a potent cancer treatment but may give rise to immune-related adverse events (irAEs). A subgroup of patients on ICIs are simultaneously immunocompromised (i.e., HIV, organ transplant recipients), which may affect their risk of developing irAEs. Conversely, the use of ICIs may influence the risk for developing HIV- or transplant-related complications. This study aims to explore the relationship between immunocompromising conditions and immunotherapy.
Methods: A single-center, retrospective chart review was performed of patients with HIV or immunosuppression following organ transplant around the time of immunotherapy. Patients not actively immunocompromised (CD4 count >500; no immunosuppressive medication) were excluded. We collected data on patient demographics, occurrence of inflammatory AEs (either GVHD or irAEs, which are difficult to distinguish), and treatment outcomes.
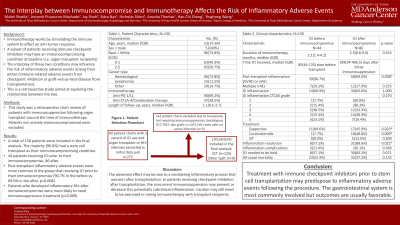
Results: A total of 155 patients were included, of which 148 (96.7%) received organ transplants [primarily stem cell (SCT)]. We compared the incidence of post-transplant inflammatory AEs among patients receiving ICI months before and after transplantation. Around 90% of patients who received ICIs pre-transplant experienced a post-transplant AE compared to 69% in those receiving ICIs after their transplant (p=0.008). Roughly 60% of the inflammatory conditions involved the GI system. Severity and complication rates did not differ between groups, but GI inflammation in the ICI-after SCT group was more likely to resolve (88.6% resolved vs. 57.1% in the ICI-before group, p=0.022). There was no significant difference in mortality between both groups.
Discussion: Our study found that patients receiving ICIs before their transplant more commonly developed inflammation post-SCT. This could be due to the ICI’s immunostimulatory effect which may predispose to future AEs and the lack of immunosuppression in this group which may facilitate a baseline subclinical inflammatory process that progresses after SCT. In contrast, patients receiving ICIs post-transplant are already on immunosuppression and are expected to have lower rates of inflammation. Such conditions are more common in the GI system with no apparent difference in clinical presentation. Our sample demonstrated favorable outcomes with high resolution rates and a minimal requirement for escalation of immunosuppression for AEs. Future studies are needed to further explore this phenomenon.
Disclosures:
Malek Shatila, MD1, Antonio Pizuorno Machado, MD2, Jay S. Shah, MD3, Sidra Naz, MD, MPH1, Nicholas Short, MD1, Anusha Thomas, MD1, Hao Chi Zhang, MD1, Yinghong Wang, MD1. P1603 - The Interplay Between Immunocompromise and Immunotherapy Affects the Risk of Inflammatory Adverse Events, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas Health Science Center, Houston, TX; 3Baylor College of Medicine, Houston, TX
Introduction: Immune checkpoint inhibitors (ICIs) are a potent cancer treatment but may give rise to immune-related adverse events (irAEs). A subgroup of patients on ICIs are simultaneously immunocompromised (i.e., HIV, organ transplant recipients), which may affect their risk of developing irAEs. Conversely, the use of ICIs may influence the risk for developing HIV- or transplant-related complications. This study aims to explore the relationship between immunocompromising conditions and immunotherapy.
Methods: A single-center, retrospective chart review was performed of patients with HIV or immunosuppression following organ transplant around the time of immunotherapy. Patients not actively immunocompromised (CD4 count >500; no immunosuppressive medication) were excluded. We collected data on patient demographics, occurrence of inflammatory AEs (either GVHD or irAEs, which are difficult to distinguish), and treatment outcomes.
Results: A total of 155 patients were included, of which 148 (96.7%) received organ transplants [primarily stem cell (SCT)]. We compared the incidence of post-transplant inflammatory AEs among patients receiving ICI months before and after transplantation. Around 90% of patients who received ICIs pre-transplant experienced a post-transplant AE compared to 69% in those receiving ICIs after their transplant (p=0.008). Roughly 60% of the inflammatory conditions involved the GI system. Severity and complication rates did not differ between groups, but GI inflammation in the ICI-after SCT group was more likely to resolve (88.6% resolved vs. 57.1% in the ICI-before group, p=0.022). There was no significant difference in mortality between both groups.
Discussion: Our study found that patients receiving ICIs before their transplant more commonly developed inflammation post-SCT. This could be due to the ICI’s immunostimulatory effect which may predispose to future AEs and the lack of immunosuppression in this group which may facilitate a baseline subclinical inflammatory process that progresses after SCT. In contrast, patients receiving ICIs post-transplant are already on immunosuppression and are expected to have lower rates of inflammation. Such conditions are more common in the GI system with no apparent difference in clinical presentation. Our sample demonstrated favorable outcomes with high resolution rates and a minimal requirement for escalation of immunosuppression for AEs. Future studies are needed to further explore this phenomenon.
Disclosures:
Malek Shatila indicated no relevant financial relationships.
Antonio Pizuorno Machado indicated no relevant financial relationships.
Jay Shah indicated no relevant financial relationships.
Sidra Naz indicated no relevant financial relationships.
Nicholas Short indicated no relevant financial relationships.
Anusha Thomas indicated no relevant financial relationships.
Hao Chi Zhang indicated no relevant financial relationships.
Yinghong Wang: ilyapharma – Consultant. IOTA – Consultant. Janssen – Consultant. MabQuest – Advisory Committee/Board Member. Sorriso – Consultant. Tillotts – Consultant.
Malek Shatila, MD1, Antonio Pizuorno Machado, MD2, Jay S. Shah, MD3, Sidra Naz, MD, MPH1, Nicholas Short, MD1, Anusha Thomas, MD1, Hao Chi Zhang, MD1, Yinghong Wang, MD1. P1603 - The Interplay Between Immunocompromise and Immunotherapy Affects the Risk of Inflammatory Adverse Events, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
