Monday Poster Session
Category: Colon
P1698 - Profound Hypovolemic Shock from Cemiplimab Induced Colitis: A Case Report
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Saeed Graham, MD
East Carolina University Brody School of Medicine
Greenville, NC
Presenting Author(s)
Saeed Graham, MD1, Stanley O. Oghoghorie, MD2, Rana Mohamed, MD1, Christin Wilkinson, MD1, Kara Regan, MD1
1East Carolina University Brody School of Medicine, Greenville, NC; 2ECU Health Medical Center, Greenville, NC
Introduction: The use of immune checkpoint inhibitors (ICI) has become ubiquitous throughout the field of oncology. By blocking natural ligands or receptors of checkpoint proteins, these drugs essentially “unleash the brakes” of the immune system, exerting an anti-tumor effect. However, these agents reserve the potential to cause severe gastrointestinal inflammation, mediated by unhinged immune activity and loss of self-tolerance. The resulting diarrhea/colitis can be life threatening. We report a case of a cutaneous squamous cell carcinoma (cSCC) survivor who developed severe diarrhea and hypovolemic shock after treatment with the ICI Cemiplimab.
Case Description/Methods: An 83-year-old navy veteran was admitted to the ICU in February 2023 for hypovolemic shock due to profound diarrhea. He had a complex history of squamous cell carcinoma (SCC) of the scalp. After an initial excision and adjuvant radiation therapy, he underwent wide local excision in 1997 for recurrence. A questionable scalp lesion prompted a repeat shave biopsy in November 2021 which demonstrated re-recurrence of SCC. Imaging for staging showed osseous invasion of the skull. His disease was deemed irresectable. Prior radiation and deep tumor invasion precluded him from radiation therapy. In March 2022 he was started on the ICI Cemiplimab. He demonstrated good response and remained in stable health until February 2023 when acute onset of severe diarrhea resulted in hypovolemic shock and acute renal failure. Stool microscopy and testing for a variety of infectious agents returned negative. He then underwent lower endoscopy with biopsy which revealed an erythematous sigmoid mucosa on gross and active colitis on histopathology. Following therapy with prednisone 1 mg/kg/day, his diarrhea resolved. He was then transferred to a lower level of care.
Discussion: Until recently, patients with cutaneous SCC (cSCC), non-eligible for curative resection or radiotherapy were limited to therapies associated with significant toxicity. Cemiplimab, an ICI approved in the USA in 2018 has shown durable clinical response and good tolerability, satisfying an otherwise unmet need in this demographic. Widespread use has unveiled unique spectra of adverse effects including life-threatening diarrhea/colitis. High grade toxicities warrant corticosteroid therapy. Endoscopic biopsy allows confirmation of the diagnosis and earlier initiation of treatment. In patients on ICI therapy with new onset gastrointestinal symptoms a diagnosis of ICI colitis should be entertained.

Disclosures:
Saeed Graham, MD1, Stanley O. Oghoghorie, MD2, Rana Mohamed, MD1, Christin Wilkinson, MD1, Kara Regan, MD1. P1698 - Profound Hypovolemic Shock from Cemiplimab Induced Colitis: A Case Report, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1East Carolina University Brody School of Medicine, Greenville, NC; 2ECU Health Medical Center, Greenville, NC
Introduction: The use of immune checkpoint inhibitors (ICI) has become ubiquitous throughout the field of oncology. By blocking natural ligands or receptors of checkpoint proteins, these drugs essentially “unleash the brakes” of the immune system, exerting an anti-tumor effect. However, these agents reserve the potential to cause severe gastrointestinal inflammation, mediated by unhinged immune activity and loss of self-tolerance. The resulting diarrhea/colitis can be life threatening. We report a case of a cutaneous squamous cell carcinoma (cSCC) survivor who developed severe diarrhea and hypovolemic shock after treatment with the ICI Cemiplimab.
Case Description/Methods: An 83-year-old navy veteran was admitted to the ICU in February 2023 for hypovolemic shock due to profound diarrhea. He had a complex history of squamous cell carcinoma (SCC) of the scalp. After an initial excision and adjuvant radiation therapy, he underwent wide local excision in 1997 for recurrence. A questionable scalp lesion prompted a repeat shave biopsy in November 2021 which demonstrated re-recurrence of SCC. Imaging for staging showed osseous invasion of the skull. His disease was deemed irresectable. Prior radiation and deep tumor invasion precluded him from radiation therapy. In March 2022 he was started on the ICI Cemiplimab. He demonstrated good response and remained in stable health until February 2023 when acute onset of severe diarrhea resulted in hypovolemic shock and acute renal failure. Stool microscopy and testing for a variety of infectious agents returned negative. He then underwent lower endoscopy with biopsy which revealed an erythematous sigmoid mucosa on gross and active colitis on histopathology. Following therapy with prednisone 1 mg/kg/day, his diarrhea resolved. He was then transferred to a lower level of care.
Discussion: Until recently, patients with cutaneous SCC (cSCC), non-eligible for curative resection or radiotherapy were limited to therapies associated with significant toxicity. Cemiplimab, an ICI approved in the USA in 2018 has shown durable clinical response and good tolerability, satisfying an otherwise unmet need in this demographic. Widespread use has unveiled unique spectra of adverse effects including life-threatening diarrhea/colitis. High grade toxicities warrant corticosteroid therapy. Endoscopic biopsy allows confirmation of the diagnosis and earlier initiation of treatment. In patients on ICI therapy with new onset gastrointestinal symptoms a diagnosis of ICI colitis should be entertained.

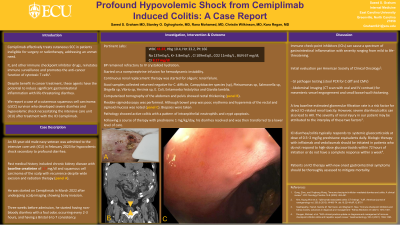
Figure: A - scalp lesion. B - Computerized tomography image of abdomen showing thickening and sigmoid colon and rectum. C- colonoscopy image showing an erythematous sigmoid colon.
Disclosures:
Saeed Graham indicated no relevant financial relationships.
Stanley Oghoghorie indicated no relevant financial relationships.
Rana Mohamed indicated no relevant financial relationships.
Christin Wilkinson indicated no relevant financial relationships.
Kara Regan indicated no relevant financial relationships.
Saeed Graham, MD1, Stanley O. Oghoghorie, MD2, Rana Mohamed, MD1, Christin Wilkinson, MD1, Kara Regan, MD1. P1698 - Profound Hypovolemic Shock from Cemiplimab Induced Colitis: A Case Report, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

