Monday Poster Session
Category: Colorectal Cancer Prevention
P1781 - Contrary to ACA Mandate for Screening Colonoscopy, Most Colonoscopy Preps Result in Out-of-Pocket Costs for Patients – A Real World Analysis From a Large Dataset
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Daniel L. Halberg, PhD
Sebela Pharmaceuticals
Braintree, MA
Presenting Author(s)
Eric D. Shah, MD, MBA, FACG1, Audrey H.. Calderwood, MD, MS2, Daniel L. Halberg, PhD3
1University of Michigan, Ann Arbor, MI; 2Dartmouth Health, Lebanon, NH; 3Sebela Pharmaceuticals, Braintree, MA
Introduction: In 2016, the Centers for Medicare & Medicaid Services (CMS) issued an Affordable Care Act (ACA) clarification regarding coverage of related costs for screening colonoscopy, concluding that bowel preparation should be covered without cost sharing, subject to “reasonable medical management.” High volume (HV), 4-liter products, first introduced in 1984, are most often offered as a formulary option at zero cost to meet this mandate. However, because of low relative tolerability and palatability associated with HV products, ~76% patients will receive an FDA unapproved over the counter (OTC) combination (~50%) or a low volume (LV) (≤ 2L) product (~26%) that may be associated with a patient co-pay. We hypothesize that most colonoscopy patients are subject to out-of-pocket costs (OPC) for bowel preparation, contrary to the ACA guidance. The goal of this study was to evaluate real world OPC for colonoscopy patients using a large prescription claims dataset and cost survey.
Methods: Prescription (Rx) level data were derived from the OPC Provider tool from IQVIA which provides real world activity for the latest 12-month claim interval. Unprojected distributions of OPC for prescription only bowel preps were generated and collated. Claims were subdivided into Cash, Commercial, Medicaid, and Medicare with cost analysis focused on commercial claims. Prescription preps were aggregated into LV and HV. Since OPC Provider only evaluates prescription costs, the OPC for OTC products was imputed by conducting a cost survey of retail pharmacies.
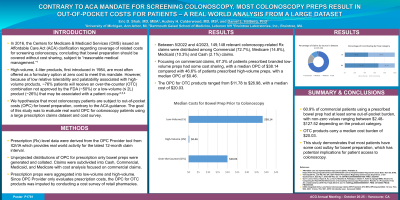
Results: Between 5/2022 and 4/2023, 149,148 relevant colonoscopy-related Rx claims were distributed among Commercial (72.7%), Medicare (14.8%), Medicaid (10.3%) and Cash (2.1%) claims. Focusing on commercial claims, 67.3% of patients prescribed branded LV had some cost sharing, with a median OPC of $38.14 compared with 46.6% of patients prescribed HV, with a median OPC of $0.46. The OPC for OTC products ranged from $11.78 to $26.98, with a median of $20.03.
Discussion: In this study of OPC costs for patients undergoing colonoscopy, 60.9% of commercial patients using a prescribed bowel prep had at least some out-of-pocket burden, with non-zero values ranging between $2.48-$127.52 depending on the product category. OTC products also carry a median cost burden of $20.03. This study demonstrates that most patients have some cost outlay for bowel preparation, which has potential implications for patient access to colonoscopy.

Disclosures:
Eric D. Shah, MD, MBA, FACG1, Audrey H.. Calderwood, MD, MS2, Daniel L. Halberg, PhD3. P1781 - Contrary to ACA Mandate for Screening Colonoscopy, Most Colonoscopy Preps Result in Out-of-Pocket Costs for Patients – A Real World Analysis From a Large Dataset, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Michigan, Ann Arbor, MI; 2Dartmouth Health, Lebanon, NH; 3Sebela Pharmaceuticals, Braintree, MA
Introduction: In 2016, the Centers for Medicare & Medicaid Services (CMS) issued an Affordable Care Act (ACA) clarification regarding coverage of related costs for screening colonoscopy, concluding that bowel preparation should be covered without cost sharing, subject to “reasonable medical management.” High volume (HV), 4-liter products, first introduced in 1984, are most often offered as a formulary option at zero cost to meet this mandate. However, because of low relative tolerability and palatability associated with HV products, ~76% patients will receive an FDA unapproved over the counter (OTC) combination (~50%) or a low volume (LV) (≤ 2L) product (~26%) that may be associated with a patient co-pay. We hypothesize that most colonoscopy patients are subject to out-of-pocket costs (OPC) for bowel preparation, contrary to the ACA guidance. The goal of this study was to evaluate real world OPC for colonoscopy patients using a large prescription claims dataset and cost survey.
Methods: Prescription (Rx) level data were derived from the OPC Provider tool from IQVIA which provides real world activity for the latest 12-month claim interval. Unprojected distributions of OPC for prescription only bowel preps were generated and collated. Claims were subdivided into Cash, Commercial, Medicaid, and Medicare with cost analysis focused on commercial claims. Prescription preps were aggregated into LV and HV. Since OPC Provider only evaluates prescription costs, the OPC for OTC products was imputed by conducting a cost survey of retail pharmacies.
Results: Between 5/2022 and 4/2023, 149,148 relevant colonoscopy-related Rx claims were distributed among Commercial (72.7%), Medicare (14.8%), Medicaid (10.3%) and Cash (2.1%) claims. Focusing on commercial claims, 67.3% of patients prescribed branded LV had some cost sharing, with a median OPC of $38.14 compared with 46.6% of patients prescribed HV, with a median OPC of $0.46. The OPC for OTC products ranged from $11.78 to $26.98, with a median of $20.03.
Discussion: In this study of OPC costs for patients undergoing colonoscopy, 60.9% of commercial patients using a prescribed bowel prep had at least some out-of-pocket burden, with non-zero values ranging between $2.48-$127.52 depending on the product category. OTC products also carry a median cost burden of $20.03. This study demonstrates that most patients have some cost outlay for bowel preparation, which has potential implications for patient access to colonoscopy.

Figure: Median Costs for Bowel Prep Prior to Colonoscopy
Disclosures:
Eric Shah: Ardelyx – Advisory Committee/Board Member, Consultant. GI Supply/Laborie – Advisory Committee/Board Member, Consultant. Mahana – Consultant. Neuraxis – Consultant. Salix – Advisory Committee/Board Member. Sanofi – Advisory Committee/Board Member, Consultant. Takeda – Consultant.
Audrey Calderwood: Dark Canyon Laboratories – Advisory Committee/Board Member.
Daniel Halberg: Sebela Pharmaceuticals – Employee.
Eric D. Shah, MD, MBA, FACG1, Audrey H.. Calderwood, MD, MS2, Daniel L. Halberg, PhD3. P1781 - Contrary to ACA Mandate for Screening Colonoscopy, Most Colonoscopy Preps Result in Out-of-Pocket Costs for Patients – A Real World Analysis From a Large Dataset, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
