Sunday Poster Session
Category: Biliary/Pancreas
P0004 - Safety and Efficacy of Liposomal Irinotecan as the Second-Line Treatment for Locally Advanced or Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials and Real-World Studies
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Shu-Yen Emily Chan, MD, MS
Weiss Memorial Hospital
Chicago, IL
Presenting Author(s)
Shu-Yen Emily Chan, MD, MS1, Kevin Sheng-Kai Ma, PhD2
1Weiss Memorial Hospital, Chicago, IL; 2Harvard T.H. Chan School of Public Health, Boston, MA
Introduction: Locally advanced or metastatic pancreatic cancer presents challenges with limited treatment options and poor prognosis. While gemcitabine-based chemotherapy has been used as the first-line therapy, liposomal irinotecan, with its longer half-life and increased area under the concentration-time curve, holds promise as a second-line therapy. This study aimed to provide evidence on the safety and efficacy of liposomal irinotecan as a second-line treatment for locally advanced or metastatic pancreatic cancer.
Methods: A systemic literature search was conducted on PubMed, Cochrane Library, EMBASE, and Web of Science databases to identify eligible clinical trials and observational studies focusing on the efficacy of liposomal irinotecan as a second-line treatment for advanced pancreatic cancer. The search encompassed studies published up to May 26th, 2023. A meta-analysis utilizing a bivariate and random-effects model was performed to derive pooled risk ratios (RRs) for essential clinical outcomes, such as overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and the incidence of grade 3 or 4 adverse events.
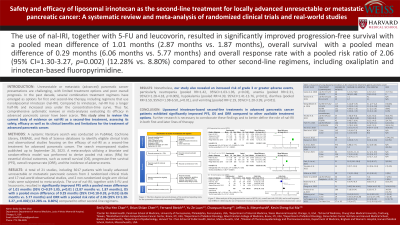
Results: A total of 15 studies, including 1,129 patients of locally advanced or metastatic pancreatic cancers from 3 randomized clinical trials and 12 real-world studies, were subjected to meta-analysis. The utilization of liposomal irinotecan resulted in a significantly improved PFS (pooled mean difference=1.38 months, 95% CI: 0.78-1.99, p< 0.0001), OS (pooled mean difference=2.06 months, 95% CI: 0.48-3.63, p< 0.0001) and ORR (pooled mean difference = 10.96 months, 95% CI: 3.7-32.5, p< 0.0001) compared to other second-line regimens. However, a significantly increased risk of grade 3 or 4 neutropenia (pooled RR=6.86, 95%CI: 2.22-21.24, p=0.0008), diarrhea (pooled RR=3.86, 95%CI: 2.16-6.92, p< 0.0001), hypokalemia (pooled RR=3.3, 95%CI: 1.23-8.89, p=0.020), and vomiting (pooled RR=3.46, 95%CI: 1.50-7.96, p=0.003) were noted.
Discussion: Liposomal irinotecan-based second-line treatments significantly improved PFS, OS and ORR in patients with advanced pancreatic cancer. Further research is necessary to corroborate these findings and ascertain the efficacy of liposomal irinotecan in both primary and subsequent treatment settings.
Disclosures:
Shu-Yen Emily Chan, MD, MS1, Kevin Sheng-Kai Ma, PhD2. P0004 - Safety and Efficacy of Liposomal Irinotecan as the Second-Line Treatment for Locally Advanced or Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials and Real-World Studies, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Weiss Memorial Hospital, Chicago, IL; 2Harvard T.H. Chan School of Public Health, Boston, MA
Introduction: Locally advanced or metastatic pancreatic cancer presents challenges with limited treatment options and poor prognosis. While gemcitabine-based chemotherapy has been used as the first-line therapy, liposomal irinotecan, with its longer half-life and increased area under the concentration-time curve, holds promise as a second-line therapy. This study aimed to provide evidence on the safety and efficacy of liposomal irinotecan as a second-line treatment for locally advanced or metastatic pancreatic cancer.
Methods: A systemic literature search was conducted on PubMed, Cochrane Library, EMBASE, and Web of Science databases to identify eligible clinical trials and observational studies focusing on the efficacy of liposomal irinotecan as a second-line treatment for advanced pancreatic cancer. The search encompassed studies published up to May 26th, 2023. A meta-analysis utilizing a bivariate and random-effects model was performed to derive pooled risk ratios (RRs) for essential clinical outcomes, such as overall survival (OS), progression-free survival (PFS), overall response rate (ORR), and the incidence of grade 3 or 4 adverse events.
Results: A total of 15 studies, including 1,129 patients of locally advanced or metastatic pancreatic cancers from 3 randomized clinical trials and 12 real-world studies, were subjected to meta-analysis. The utilization of liposomal irinotecan resulted in a significantly improved PFS (pooled mean difference=1.38 months, 95% CI: 0.78-1.99, p< 0.0001), OS (pooled mean difference=2.06 months, 95% CI: 0.48-3.63, p< 0.0001) and ORR (pooled mean difference = 10.96 months, 95% CI: 3.7-32.5, p< 0.0001) compared to other second-line regimens. However, a significantly increased risk of grade 3 or 4 neutropenia (pooled RR=6.86, 95%CI: 2.22-21.24, p=0.0008), diarrhea (pooled RR=3.86, 95%CI: 2.16-6.92, p< 0.0001), hypokalemia (pooled RR=3.3, 95%CI: 1.23-8.89, p=0.020), and vomiting (pooled RR=3.46, 95%CI: 1.50-7.96, p=0.003) were noted.
Discussion: Liposomal irinotecan-based second-line treatments significantly improved PFS, OS and ORR in patients with advanced pancreatic cancer. Further research is necessary to corroborate these findings and ascertain the efficacy of liposomal irinotecan in both primary and subsequent treatment settings.
Disclosures:
Shu-Yen Emily Chan indicated no relevant financial relationships.
Kevin Sheng-Kai Ma indicated no relevant financial relationships.
Shu-Yen Emily Chan, MD, MS1, Kevin Sheng-Kai Ma, PhD2. P0004 - Safety and Efficacy of Liposomal Irinotecan as the Second-Line Treatment for Locally Advanced or Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis of Randomized Clinical Trials and Real-World Studies, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
