Sunday Poster Session
Category: Biliary/Pancreas
P0134 - The Dark Side of Semaglutide: Discrete Instances of Acalculous Cholecystitis and Acute Pancreatitis in the Same Patient Due to Continued Use of GLP-1 Agonist
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Ruona Ebiai, MD
Ochsner Clinic Foundation
New Orleans, LA
Presenting Author(s)
Ruona Ebiai, MD1, James Gore, DO1, Bradley S. Kapten, MD2
1Ochsner Clinic Foundation, New Orleans, LA; 2Ascension Providence Hospital/ Michigan State University College of Human Medicine, Southfield, MI
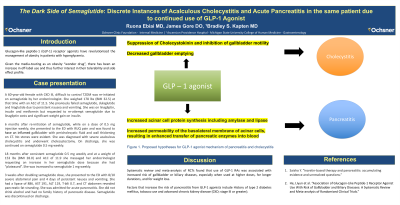
Introduction: Glucagon-like peptide-1 (GLP-1) receptor agonists have revolutionized management of obesity in patients with hyperglycemia. Given it’s media-touting as an obesity “wonder drug”, there has been an increase in off-label use and thus further interest in their tolerability and side effect profile. We present a case of semaglutide use leading to separate episodes of acute acalculous cholecystitis and then acute pancreatitis in the same patient.
Case Description/Methods: A 60-year-old female with difficult to control T2DM was re-initiated on semaglutide by her endocrinologist. She weighed 178 lbs (BMI 32.5) at that time with an A1C of 11.5. She previously failed semaglutide, dulaglutide and liraglutide due to persistent nausea and vomiting. She was currently on linagliptin, insulin and metformin but requested to re-attempt semaglutide due to linagliptin costs and significant weight gain on insulin.
6 months after re-initiation of semaglutide and while on a dose of 0.5 mg injection weekly, she presented to the ED with RUQ pain and was found to have inflamed gallbladder with pericholecystic fluid and wall thickening on CT. No stones were evident. She was diagnosed with severe acalculous cholecystitis and underwent cholecystectomy. On discharge, she was continued on semaglutide 0.5 mg weekly.
18 months after consistent semaglutide 0.5 mg weekly and at a weight of 153 lbs (BMI 28.0) and A1C of 11.9 she messaged her endocrinologist requesting an increase in her semaglutide dose because she had “plateaued”. She was increased to semaglutide 1 mg weekly.
3 weeks after doubling semaglutide dose, she presented to the ED with 8/10 severe abdominal pain and 4 days of persistent nausea and vomiting. She had a lipase of 886, AST 191, ALT 119, Tbili 0.7, and CT abdomen revealed pancreatic fat stranding. She was admitted for acute pancreatitis. She did not drink alcohol and had no family history of pancreatic disease. Semaglutide was discontinued on discharge.
Discussion: With GLP-1 agonists being touted as a sure-fire answer to weight difficulties, there has been a lot of excitement surrounding these traditionally less popular anti-hyperglycemic medications. It is important to accurately characterize the risk of adverse effects for patients prior to initiation. For patients without obvious risk factors for gallbladder or pancreatic disease, this task is significantly more difficult and there should be a low threshold to identify these medications as the culprit when there is no more glaring explanation.
Disclosures:
Ruona Ebiai, MD1, James Gore, DO1, Bradley S. Kapten, MD2. P0134 - The Dark Side of Semaglutide: Discrete Instances of Acalculous Cholecystitis and Acute Pancreatitis in the Same Patient Due to Continued Use of GLP-1 Agonist, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Ochsner Clinic Foundation, New Orleans, LA; 2Ascension Providence Hospital/ Michigan State University College of Human Medicine, Southfield, MI
Introduction: Glucagon-like peptide-1 (GLP-1) receptor agonists have revolutionized management of obesity in patients with hyperglycemia. Given it’s media-touting as an obesity “wonder drug”, there has been an increase in off-label use and thus further interest in their tolerability and side effect profile. We present a case of semaglutide use leading to separate episodes of acute acalculous cholecystitis and then acute pancreatitis in the same patient.
Case Description/Methods: A 60-year-old female with difficult to control T2DM was re-initiated on semaglutide by her endocrinologist. She weighed 178 lbs (BMI 32.5) at that time with an A1C of 11.5. She previously failed semaglutide, dulaglutide and liraglutide due to persistent nausea and vomiting. She was currently on linagliptin, insulin and metformin but requested to re-attempt semaglutide due to linagliptin costs and significant weight gain on insulin.
6 months after re-initiation of semaglutide and while on a dose of 0.5 mg injection weekly, she presented to the ED with RUQ pain and was found to have inflamed gallbladder with pericholecystic fluid and wall thickening on CT. No stones were evident. She was diagnosed with severe acalculous cholecystitis and underwent cholecystectomy. On discharge, she was continued on semaglutide 0.5 mg weekly.
18 months after consistent semaglutide 0.5 mg weekly and at a weight of 153 lbs (BMI 28.0) and A1C of 11.9 she messaged her endocrinologist requesting an increase in her semaglutide dose because she had “plateaued”. She was increased to semaglutide 1 mg weekly.
3 weeks after doubling semaglutide dose, she presented to the ED with 8/10 severe abdominal pain and 4 days of persistent nausea and vomiting. She had a lipase of 886, AST 191, ALT 119, Tbili 0.7, and CT abdomen revealed pancreatic fat stranding. She was admitted for acute pancreatitis. She did not drink alcohol and had no family history of pancreatic disease. Semaglutide was discontinued on discharge.
Discussion: With GLP-1 agonists being touted as a sure-fire answer to weight difficulties, there has been a lot of excitement surrounding these traditionally less popular anti-hyperglycemic medications. It is important to accurately characterize the risk of adverse effects for patients prior to initiation. For patients without obvious risk factors for gallbladder or pancreatic disease, this task is significantly more difficult and there should be a low threshold to identify these medications as the culprit when there is no more glaring explanation.
Disclosures:
Ruona Ebiai indicated no relevant financial relationships.
James Gore indicated no relevant financial relationships.
Bradley Kapten indicated no relevant financial relationships.
Ruona Ebiai, MD1, James Gore, DO1, Bradley S. Kapten, MD2. P0134 - The Dark Side of Semaglutide: Discrete Instances of Acalculous Cholecystitis and Acute Pancreatitis in the Same Patient Due to Continued Use of GLP-1 Agonist, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
