Sunday Poster Session
Category: Colon
P0167 - Immune Checkpoint Inhibitor-Associated Gastrointestinal Adverse Events in Patients With Colorectal Cancer

Antonio Pizuorno Machado, MD
University of Texas Health Science Center
Houston, TX
Presenting Author(s)
1University of Texas Health Science Center, Houston, TX; 2University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Immune checkpoint inhibitors (ICI) are currently employed for the management of microsatellite instability-high (MSI-H) tumors with success. While immune checkpoint inhibitor related colitis is a very frequent and devastating immune related adverse event (irAE) with the use of these agents, the incidence and characteristics of this inflammatory toxicity in patients with colorectal cancers has not been examined. We aimed to describe the characteristics and clinical profile of patients diagnosed with luminal gastrointestinal irAE in patients treated with ICI for colorectal cancer in a tertiary cancer care center.
Methods: This is a retrospective analysis that included adult cancer patients diagnosed with colorectal cancer that received ICI between 6/1/2014 and 12/31/2022. We report data on those that developed colitis secondary to irAE up to 3 months after the last dose of ICI confirmed by laboratory and/or imaging report. We included patients’ demographic characteristics, oncologic profile and outcomes as well as clinical course, endoscopic features as well as treatment and outcomes in terms of luminal gastrointestinal irAEs.
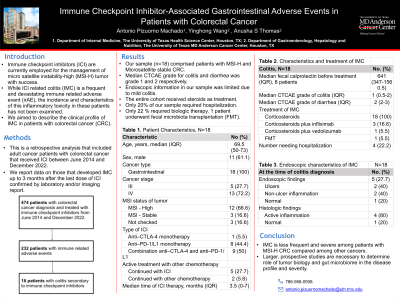
Results: Our sample (n=18) comprised primarily of Caucasian (88.8%) males (61.1%) where in, the incidence of gastrointestinal irAE was 8.3%. The majority of these patients received combination therapy with anti-PD1/L1 and CTLA-4 (50%). 66.6 % received ICI for MSI-H colorectal cancer. 11.1% of our sample were noted to have a second cancer-melanoma. Colitis generally had a median CTCAE grade of 1, and diarrhea of 2 (5.8% had grade ≥3 colitis). Only 5 patients underwent endoscopic evaluation of whom, 2 had ulcerative inflammation necessitating use of selective immunosuppressive therapy with biologics. 61.1% had to withhold cancer treatment due toxicity. In regards to other gastrointestinal irAE among this population, 41.4% and 5.8% were noted to have liver and pancreas toxicity with a median CTCAE grade of severity 2. The most common treatment for the toxicities were steroids.
Discussion: Gastrointestinal irAEs appear to be less frequent at lower severity among patients with MSI-H colorectal cancer after checkpoint inhibitors exposure compared to the overall incidence of the same among other cancers reported in literature. Larger prospective studies are necessary to determine the role of tumor biology and the gut microbiome in the disease profile and severity of immune related adverse events of the GI organ system.
Table 1. Characteristics of gastrointestinal irAE in patients with colorectal cancer and Endoscopy-related characteristics for patients diagnosed with colitis, N=18 |
|
Colitis, N=18 | No. (%) |
Symptoms |
|
Diarrhea | 18 (100) |
Abdominal pain | 18 (100) |
Nausea/vomiting | 1 (5.5) |
Median fecal calprotectin before treatment (IQR), 8 patients | 641 (347-1560.5) |
Median CTCAE grade of colitis (IQR) | 1 (0.5-2) |
Median CTCAE grade of diarrhea (IQR) | 2 (2-3) |
Grade I diarrhea | 10 (55.5) |
Grade II diarrhea | 6 (33.3) |
Grade III and IV diarrhea | 2 (5.8) |
Hospitalization required | 4 (22.2) |
Cancer treatment withheld due to toxicity | 11 (61.1) |
All cause mortality | 7 (38.8) |
At the time of colitis diagnosis | No. (%) |
Endoscopic findings | 5 (27.7) |
Ulcers | 2 (40) |
Non-ulcer inflammation | 2 (40) |
Normal | 1 (20) |
Histologic findings |
|
Active inflammation | 4 (80) |
Normal | 1 (20) |
Treatment of IMC |
|
Corticosteroids | 18 (100) |
Corticosteroids plus infliximab only | 3 (16.6) |
Corticosteroids plus vedolizumab only | 1 (5.5) |
Corticosteroids plus both infliximab and vedolizumab | 0 (0) |
Corticosteroids plus ustekinumab add-on | 0 (0) |
FMT | 1 (5.5) |
Complications of IMC | 1 (5.5) |
Perforation | 1 (5.5) |
Abbreviations: CTCAE v5, Common Terminology Criteria for Adverse Events version 5; ICI, immune checkpoint inhibitor; IMC, immune-mediated colitis; IQR, interquartile range; TNF, tumor necrosis factor; FMT, fecal microbiota transplantation; irAE, immune related adverse event | |
Disclosures:
Antonio Pizuorno Machado, MD1, Yinghong Wang, MD2, Anusha Thomas, MD2. P0167 - Immune Checkpoint Inhibitor-Associated Gastrointestinal Adverse Events in Patients With Colorectal Cancer, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
