Sunday Poster Session
Category: Colon
P0318 - Ocrelizumab Induced Colitis: A Rare Clinical Entity Mimicking Cytomegalovirus Colitis
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Aishwarya Thakurdesai, MBBS, MD
University of Louisville School of Medicine
Louisville, KY
Presenting Author(s)
Aishwarya Thakurdesai, MD1, Navroop Nagra, MD1, Dipendra Parajuli, MD2
1University of Louisville, Louisville, KY; 2University of Louisville School of Medicine, Louisville, KY
Introduction: Ocrelizumab is an anti- CD20 monoclonal antibody used in treatment of Multiple Sclerosis (MS). It works by depleting B cells, which can in turn lead to CD4+ T cell proliferation and increased production of proinflammatory cytokines, disrupting gut homeostasis and causing colitis in < 1 % cases. Herein, we present a case of ocrelizumab induced colitis with endoscopic appearance of cytomegalovirus (CMV) colitis.
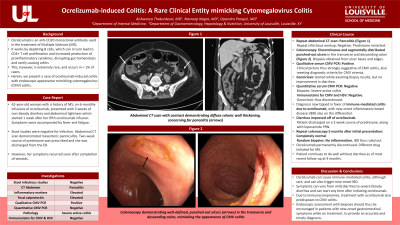
Case Description/Methods: 62-year-old woman with history of MS, on 6-monthly infusions of ocrelizumab, presented with 3 weeks of non-bloody diarrhea and abdominal tightness which started 1 week after her fifth ocrelizumab infusion. Stool studies were negative for infection. Abdominal CT scan demonstrated mesenteric panniculitis. Two week course of prednisone was prescribed and she was discharged from ER. However, her symptoms recurred soon after completion of steroids. Repeat abdominal CT showed pancolitis. Repeat infectious work up was negative. Prednisone was restarted. Colonoscopy was performed which revealed discontinuous and segmentally distributed punched out ulcers in transverse and descending colon (Figure 1). Biopsies were taken from ulcer base and edges. Her clinical picture was thus strongly suggestive of CMV colitis. Ganciclovir was started while awaiting biopsy results, without improvement. Quantitative serum CMV PCR came back negative. Biopsies showed severe active colitis, but immunostains for CMV and HSV were negative. Ganciclovir was thus discontinued. The diagnosis had now tipped in favor of immune-mediated colitis due to ocrelizumab, with new onset inflammatory bowel disease (IBD) also on the differential. Her diarrhea improved and she was discharged on 2-week course of prednisone, along with as needed loperamide. Repeat colonoscopy performed 5 months after her initial presentation was completely normal and random biopsies taken showed no inflammation, ruling out IBD. Ocrelizumab was thus discontinued, and a different drug was initiated for MS. The patient continues to do well without diarrhea as of most recent follow up at 9 months.
Discussion: Ocrelizumab can cause immune mediated colitis and can trigger new onset IBD. Symptoms can vary from mild diarrhea to severe bloody diarrhea and can start at any time after initiating ocrelizumab. It also predisposes to CMV colitis; therefore, endoscopic assessment with biopsies should be encouraged in patients with new onset gastrointestinal symptoms while on treatment, to provide an accurate and timely diagnosis.

Disclosures:
Aishwarya Thakurdesai, MD1, Navroop Nagra, MD1, Dipendra Parajuli, MD2. P0318 - Ocrelizumab Induced Colitis: A Rare Clinical Entity Mimicking Cytomegalovirus Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Louisville, Louisville, KY; 2University of Louisville School of Medicine, Louisville, KY
Introduction: Ocrelizumab is an anti- CD20 monoclonal antibody used in treatment of Multiple Sclerosis (MS). It works by depleting B cells, which can in turn lead to CD4+ T cell proliferation and increased production of proinflammatory cytokines, disrupting gut homeostasis and causing colitis in < 1 % cases. Herein, we present a case of ocrelizumab induced colitis with endoscopic appearance of cytomegalovirus (CMV) colitis.
Case Description/Methods: 62-year-old woman with history of MS, on 6-monthly infusions of ocrelizumab, presented with 3 weeks of non-bloody diarrhea and abdominal tightness which started 1 week after her fifth ocrelizumab infusion. Stool studies were negative for infection. Abdominal CT scan demonstrated mesenteric panniculitis. Two week course of prednisone was prescribed and she was discharged from ER. However, her symptoms recurred soon after completion of steroids. Repeat abdominal CT showed pancolitis. Repeat infectious work up was negative. Prednisone was restarted. Colonoscopy was performed which revealed discontinuous and segmentally distributed punched out ulcers in transverse and descending colon (Figure 1). Biopsies were taken from ulcer base and edges. Her clinical picture was thus strongly suggestive of CMV colitis. Ganciclovir was started while awaiting biopsy results, without improvement. Quantitative serum CMV PCR came back negative. Biopsies showed severe active colitis, but immunostains for CMV and HSV were negative. Ganciclovir was thus discontinued. The diagnosis had now tipped in favor of immune-mediated colitis due to ocrelizumab, with new onset inflammatory bowel disease (IBD) also on the differential. Her diarrhea improved and she was discharged on 2-week course of prednisone, along with as needed loperamide. Repeat colonoscopy performed 5 months after her initial presentation was completely normal and random biopsies taken showed no inflammation, ruling out IBD. Ocrelizumab was thus discontinued, and a different drug was initiated for MS. The patient continues to do well without diarrhea as of most recent follow up at 9 months.
Discussion: Ocrelizumab can cause immune mediated colitis and can trigger new onset IBD. Symptoms can vary from mild diarrhea to severe bloody diarrhea and can start at any time after initiating ocrelizumab. It also predisposes to CMV colitis; therefore, endoscopic assessment with biopsies should be encouraged in patients with new onset gastrointestinal symptoms while on treatment, to provide an accurate and timely diagnosis.

Figure: Punched out ulcers in colon
Disclosures:
Aishwarya Thakurdesai indicated no relevant financial relationships.
Navroop Nagra indicated no relevant financial relationships.
Dipendra Parajuli indicated no relevant financial relationships.
Aishwarya Thakurdesai, MD1, Navroop Nagra, MD1, Dipendra Parajuli, MD2. P0318 - Ocrelizumab Induced Colitis: A Rare Clinical Entity Mimicking Cytomegalovirus Colitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
