Sunday Poster Session
Category: Colorectal Cancer Prevention
P0331 - Improving Colorectal Cancer Screening Rates With Multitarget Stool DNA Testing by Implementing a Comprehensive Program of Tailored Patient Navigation in an Inner-City Population
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
- EC
Edward Cytryn, MD
Icahn School of Medicine at Mount Sinai
New York, New York
Presenting Author(s)
Edward Cytryn, MD, Zachary Stauber, MD, MPH, Kayla Jaeckel, MPH, RD, CDCES, Nikita Barai, MD, Mary B. Fishman, MD, Pascale M. White, MD, MBA, MS, Lina Jandorf, MA, Steven H. Itzkowitz, MD, Kyle M. Koster, MD, MS
Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Multi-target stool DNA (mt-sDNA) test is a home-based colorectal cancer (CRC) screening test and an effective option for those preferring a non-invasive modality, including never-screened patients. At our large academic primary care clinic in New York City serving a vulnerable population, the impact of mt-sDNA has been limited by poor kit return rates and inadequate sample collection, despite built-in navigation support available with the test. Tailored patient navigation (TPN) is an effective intervention to improve cancer screening adherence. We hypothesized that implementing TPN for mt-sDNA screening would improve kit return and adequate sample collection.
Methods: All patients aged 45 to 75 referred by their provider for mt-sDNA testing were included. A patient navigator (PN) was trained in mt-sDNA workflows. The PN reviewed all new orders daily, verified delivery information, and outreached patients at specified intervals to support kit return. Baseline 60-day kit return rates were calculated during a 6-month “run-in period” before PN onboarding and compared to the ongoing intervention period. Inadequate sample rates were calculated on all completed tests (Microsoft Excel). Rates were compared using 2-tail mid-P exact test (OpenEpi). A p-value less than 0.05 was considered statistically significant. A 20% increase in completion rate and 30% decrease in inadequate sample rate were considered clinically significant.
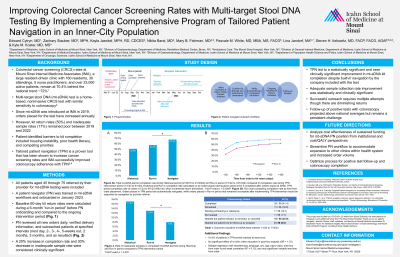
Results: The PN successfully contacted 84.9% of patients over 4 months. 60-day kit return improved from 51.9% (418 of 805) in the run-in period to 64.9% (163 of 251) in the intervention period (p=0.0003, RR 1.25, 95% CI 1.12-1.40), a statistically and clinically significant increase. Inadequate sample collection decreased from 13.2% to 8.4% (p= 0.059, RR 0.64, 95% CI 0.39-1.03), which was clinically significant.
Discussion: After implementing a novel patient navigation intervention for home-based CRC screening, patients had a statistically and clinically significant increase in test completion rates. Patients also submitted fewer inadequate samples, which was clinically though not statistically significant, likely due to insufficient power. Our data indicate that there is benefit to TPN for mt-sDNA screening in vulnerable populations compared to standard practice. Ongoing work will determine if TPN can further improve kit return and sample adequacy and assess its cost-effectiveness and impact on CRC screening rates overall.
Disclosures:
Edward Cytryn, MD, Zachary Stauber, MD, MPH, Kayla Jaeckel, MPH, RD, CDCES, Nikita Barai, MD, Mary B. Fishman, MD, Pascale M. White, MD, MBA, MS, Lina Jandorf, MA, Steven H. Itzkowitz, MD, Kyle M. Koster, MD, MS. P0331 - Improving Colorectal Cancer Screening Rates With Multitarget Stool DNA Testing by Implementing a Comprehensive Program of Tailored Patient Navigation in an Inner-City Population, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: Multi-target stool DNA (mt-sDNA) test is a home-based colorectal cancer (CRC) screening test and an effective option for those preferring a non-invasive modality, including never-screened patients. At our large academic primary care clinic in New York City serving a vulnerable population, the impact of mt-sDNA has been limited by poor kit return rates and inadequate sample collection, despite built-in navigation support available with the test. Tailored patient navigation (TPN) is an effective intervention to improve cancer screening adherence. We hypothesized that implementing TPN for mt-sDNA screening would improve kit return and adequate sample collection.
Methods: All patients aged 45 to 75 referred by their provider for mt-sDNA testing were included. A patient navigator (PN) was trained in mt-sDNA workflows. The PN reviewed all new orders daily, verified delivery information, and outreached patients at specified intervals to support kit return. Baseline 60-day kit return rates were calculated during a 6-month “run-in period” before PN onboarding and compared to the ongoing intervention period. Inadequate sample rates were calculated on all completed tests (Microsoft Excel). Rates were compared using 2-tail mid-P exact test (OpenEpi). A p-value less than 0.05 was considered statistically significant. A 20% increase in completion rate and 30% decrease in inadequate sample rate were considered clinically significant.
Results: The PN successfully contacted 84.9% of patients over 4 months. 60-day kit return improved from 51.9% (418 of 805) in the run-in period to 64.9% (163 of 251) in the intervention period (p=0.0003, RR 1.25, 95% CI 1.12-1.40), a statistically and clinically significant increase. Inadequate sample collection decreased from 13.2% to 8.4% (p= 0.059, RR 0.64, 95% CI 0.39-1.03), which was clinically significant.
Discussion: After implementing a novel patient navigation intervention for home-based CRC screening, patients had a statistically and clinically significant increase in test completion rates. Patients also submitted fewer inadequate samples, which was clinically though not statistically significant, likely due to insufficient power. Our data indicate that there is benefit to TPN for mt-sDNA screening in vulnerable populations compared to standard practice. Ongoing work will determine if TPN can further improve kit return and sample adequacy and assess its cost-effectiveness and impact on CRC screening rates overall.
Disclosures:
Edward Cytryn: Exact Sciences – Grant/Research Support.
Zachary Stauber: Exact Sciences – Grant/Research Support.
Kayla Jaeckel: Exact Sciences – Grant/Research Support.
Nikita Barai: Exact Sciences – Grant/Research Support.
Mary Fishman: Exact Sciences – Grant/Research Support.
Pascale White: Exact Sciences – Grant/Research Support.
Lina Jandorf: Exact Sciences – Grant/Research Support.
Steven Itzkowitz: Exact Sciences – Grant/Research Support.
Kyle Koster: Exact Sciences – Grant/Research Support.
Edward Cytryn, MD, Zachary Stauber, MD, MPH, Kayla Jaeckel, MPH, RD, CDCES, Nikita Barai, MD, Mary B. Fishman, MD, Pascale M. White, MD, MBA, MS, Lina Jandorf, MA, Steven H. Itzkowitz, MD, Kyle M. Koster, MD, MS. P0331 - Improving Colorectal Cancer Screening Rates With Multitarget Stool DNA Testing by Implementing a Comprehensive Program of Tailored Patient Navigation in an Inner-City Population, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
