Sunday Poster Session
Category: Colorectal Cancer Prevention
P0366 - Performance of Colon and Rectal Cancer Detection with a Multi-Cancer Early Detection (MCED) Blood Test
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Matthew Margolis, MS
GRAIL, LLC
Menlo Park, CA
Presenting Author(s)
Award: Presidential Poster Award
Matthew Margolis, MS1, Kathryn N. Kurtzman, MD2, Cheng Chen, PhD3, Jordan J.. Karlitz, MD, FACG4
1GRAIL, LLC, Menlo Park, CA; 2GRAIL, Menlo Park, CA; 3GRAIL, Inc., Menlo Park, CA; 4GRAIL, LLC, Denver, CO
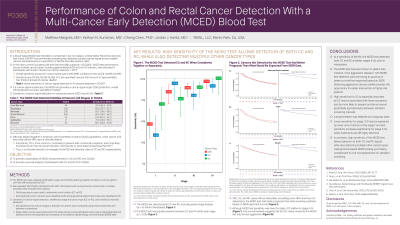
Introduction: A blood-based MCED test intended to complement, but not replace, recommended USPSTF screening can detect a cancer signal for multiple cancers simultaneously at a specificity of 99.5% (low false positive rate). In the case-control Circulating Cell-free Genome Atlas substudy 3 (CCGA3), MCED test performance across multiple cancer types was evaluated (Table 1). Sensitivity of cancer signal detection (CSD) for colorectal cancer (CRC) was 82.0%. As colon cancer (CC) and rectal cancer (RC) can be clinically distinct (CC is more common than RC, may be more likely to present with no/minimal symptoms, and can develop as interval cancers between colonoscopy or stool-based screenings), we provide a subanalysis of MCED test performance in CC and RC from CCGA3.
Methods: The MCED test uses a targeted methylation assay and machine learning classifier to detect a cancer signal in cell-free DNA and predict its origin.. We evaluated 197 CCGA3 participants with CRC and used pathology reports to determine tumor location (CC vs RC). Sensitivity of CSD stratified by stage and cancer type (CC vs RC) and overall survival were assessed.
Results: There was no significant difference in sensitivity between CC and RC across all stages (p≥0.05) (Figure 1). There was no significant difference in sensitivity for CC between stages II, III and IV (p≥0.05) and a significant difference at stage I (p< 0.05). No significant difference in sensitivity for RC was observed across stages (Figure 1). CRC and CC not detected by the MCED test had significantly better survival than those detected (Figure; p=0.0038 and p=0.014, respectively); however, no significant difference was found in RC (p=0.11).
Discussion: At a specificity of 99.5%, the MCED test can detect both CC and RC at earlier stages (prior to metastases). It has been shown to detect less-indolent, more aggressive disease (associated with worse survival), allowing opportunity for earlier intervention in higher-risk patients. High sensitivity for CC is especially useful as CC may be associated with fewer symptoms and more likely to present as interval cancers (potentially asymptomatic) between standard screening intervals. A study limitation is relatively low subgroup sizes. Lower sensitivity for stage I CC is explained by shallower depth of tumor invasion. In summary, high sensitivity of this MCED test allows detection of both CC and RC signals, including in those who may not be up to date with standard screening, while also detecting multiple other cancer types.

Disclosures:
Matthew Margolis, MS1, Kathryn N. Kurtzman, MD2, Cheng Chen, PhD3, Jordan J.. Karlitz, MD, FACG4. P0366 - Performance of Colon and Rectal Cancer Detection with a Multi-Cancer Early Detection (MCED) Blood Test, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Matthew Margolis, MS1, Kathryn N. Kurtzman, MD2, Cheng Chen, PhD3, Jordan J.. Karlitz, MD, FACG4
1GRAIL, LLC, Menlo Park, CA; 2GRAIL, Menlo Park, CA; 3GRAIL, Inc., Menlo Park, CA; 4GRAIL, LLC, Denver, CO
Introduction: A blood-based MCED test intended to complement, but not replace, recommended USPSTF screening can detect a cancer signal for multiple cancers simultaneously at a specificity of 99.5% (low false positive rate). In the case-control Circulating Cell-free Genome Atlas substudy 3 (CCGA3), MCED test performance across multiple cancer types was evaluated (Table 1). Sensitivity of cancer signal detection (CSD) for colorectal cancer (CRC) was 82.0%. As colon cancer (CC) and rectal cancer (RC) can be clinically distinct (CC is more common than RC, may be more likely to present with no/minimal symptoms, and can develop as interval cancers between colonoscopy or stool-based screenings), we provide a subanalysis of MCED test performance in CC and RC from CCGA3.
Methods: The MCED test uses a targeted methylation assay and machine learning classifier to detect a cancer signal in cell-free DNA and predict its origin.. We evaluated 197 CCGA3 participants with CRC and used pathology reports to determine tumor location (CC vs RC). Sensitivity of CSD stratified by stage and cancer type (CC vs RC) and overall survival were assessed.
Results: There was no significant difference in sensitivity between CC and RC across all stages (p≥0.05) (Figure 1). There was no significant difference in sensitivity for CC between stages II, III and IV (p≥0.05) and a significant difference at stage I (p< 0.05). No significant difference in sensitivity for RC was observed across stages (Figure 1). CRC and CC not detected by the MCED test had significantly better survival than those detected (Figure; p=0.0038 and p=0.014, respectively); however, no significant difference was found in RC (p=0.11).
Discussion: At a specificity of 99.5%, the MCED test can detect both CC and RC at earlier stages (prior to metastases). It has been shown to detect less-indolent, more aggressive disease (associated with worse survival), allowing opportunity for earlier intervention in higher-risk patients. High sensitivity for CC is especially useful as CC may be associated with fewer symptoms and more likely to present as interval cancers (potentially asymptomatic) between standard screening intervals. A study limitation is relatively low subgroup sizes. Lower sensitivity for stage I CC is explained by shallower depth of tumor invasion. In summary, high sensitivity of this MCED test allows detection of both CC and RC signals, including in those who may not be up to date with standard screening, while also detecting multiple other cancer types.

Figure: Figure 1.Sensitivity of detection and survival of CRC, CC and RC in CCGA3.
Sensitivities across stages or between colon and rectal cancer were compared using Fisher’s exact test with pairwise comparisons.
Rectosigmoid junction cancers were classified as rectal cancer and appendiceal adenocarcinomas were classified as colon cancer.
Kaplan-Meier curves were produced and survival was compared using a log-rank test.
Sensitivities across stages or between colon and rectal cancer were compared using Fisher’s exact test with pairwise comparisons.
Rectosigmoid junction cancers were classified as rectal cancer and appendiceal adenocarcinomas were classified as colon cancer.
Kaplan-Meier curves were produced and survival was compared using a log-rank test.
Table: Table 1. Examples of Gastrointestinal cancers detected by MCED (All Stages) in CCGA3.
Sensitivity of cancer signal detection stratified by gastrointestinal cancers with 95% CI (Full list of cancer types detected and corresponding CSD sensitivity previously reported in Klein E et al. Ann Oncol. 2021;32(9):1167-1177.)
*CRC subanalysis performed in this analysis.
CI = confidence interval; CSD = cancer signal detection
Sensitivity of cancer signal detection stratified by gastrointestinal cancers with 95% CI (Full list of cancer types detected and corresponding CSD sensitivity previously reported in Klein E et al. Ann Oncol. 2021;32(9):1167-1177.)
*CRC subanalysis performed in this analysis.
CI = confidence interval; CSD = cancer signal detection
Disclosures:
Matthew Margolis: Foundation Medicine – Employee, Intellectual Property/Patents, Stock Options, Stock-publicly held company(excluding mutual/index funds). GRAIL – Employee, Stock-publicly held company(excluding mutual/index funds).
Kathryn Kurtzman: GRAIL, LLC – Employee, Stock Options. Illumina – Stock Options.
Cheng Chen indicated no relevant financial relationships.
Jordan Karlitz: GastroGirl/GI OnDEMAND – Consultant, Owner/Ownership Interest. GRAIL – Employee.
Matthew Margolis, MS1, Kathryn N. Kurtzman, MD2, Cheng Chen, PhD3, Jordan J.. Karlitz, MD, FACG4. P0366 - Performance of Colon and Rectal Cancer Detection with a Multi-Cancer Early Detection (MCED) Blood Test, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

