Sunday Poster Session
Category: Esophagus
P0392 - Dupilumab Improves Dysphagia Symptoms in Adolescent and Adult Patients With Eosinophilic Esophagitis
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
- ED
Evan S. Dellon, MD, MPH
University of North Carolina School of Medicine
Chapel Hill, North Carolina
Presenting Author(s)
Award: Presidential Poster Award
Evan S. Dellon, MD, MPH1, Marc E. Rothenberg, MD, PhD2, Albert J. Bredenoord, MD3, Ikuo Hirano, MD4, Mirna Chehade, MD, MPH5, Zhen Chen, PhD, MPH, MS, MBA6, Juby A. Jacob-Nara, MD, DHSc, MPH, MBA7, Amr Radwan, MBBCh, MA6, Sarette T. Tilton, PharmD7, Eilish McCann, PhD6
1University of North Carolina School of Medicine, Chapel Hill, NC; 2Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH; 3Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Feinberg School of Medicine, Northwestern University, Chicago, IL; 5Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai, New York, NY; 6Regeneron Pharmaceuticals Inc., Tarrytown, NY; 7Sanofi, Bridgewater, NJ
Introduction: Dysphagia is the most common symptom of eosinophilic esophagitis (EoE) in adolescents and adults, but limited data exist regarding the thresholds for clinically relevant symptom response.
In the three-part phase 3 LIBERTY EoE TREET study (NCT03633617), dupilumab 300 mg weekly (qw) an antibody against the shared receptor component for interleukin IL-4 and IL-13, histologic, symptomatic, endoscopic, and molecular aspects of disease at 24 weeks versus placebo, with effects sustained up to 52 weeks in adult and adolescent patients with EoE. We aimed to evaluate the proportion of patients with EoE in Parts A and B of the LIBERTY EoE TREET study achieving a range of response thresholds in the Dysphagia Symptom Questionnaire (DSQ) biweekly total score, a validated patient-reported outcome.
Methods: Patients were randomized 1:1 to receive dupilumab 300 mg qw or placebo (Part A, n=42/39; Part B, n=80/79). For this secondary analysis, endpoints were proportion of patients achieving a ≥25%, ≥50%, ≥75%, or =100% reduction in DSQ total score at Week 24, calculated based on daily questionnaire responses over the 14-days prior to Week 24. Responders at each threshold were compared between dupilumab and placebo.
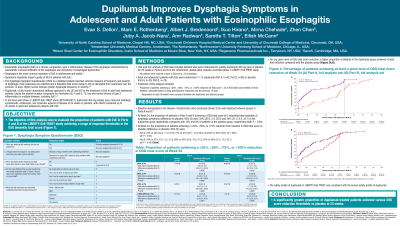
Results: The proportions of patients achieving a ≥25%, ≥50%, ≥75%, or =100% reduction from BL in DSQ total score for dupilumab versus placebo at Week 24 were:
Part A: 76%/44%; 71%/31%; 62%/23%; and 33%/13% (all P< 0.05 versus placebo). Part B: 78%/61%; 69%/43%; 55%/28%; and 29%/8% (all P< 0.05 versus placebo; Figure)
Discussion: A significantly greater proportion of dupilumab-treated patients had a reduction in DSQ score versus placebo at 24 weeks. Of these levels, it remains to be determined what DSQ response threshold optimally correlates with histologic, endoscopic, and molecular responses.

Disclosures:
Evan S. Dellon, MD, MPH1, Marc E. Rothenberg, MD, PhD2, Albert J. Bredenoord, MD3, Ikuo Hirano, MD4, Mirna Chehade, MD, MPH5, Zhen Chen, PhD, MPH, MS, MBA6, Juby A. Jacob-Nara, MD, DHSc, MPH, MBA7, Amr Radwan, MBBCh, MA6, Sarette T. Tilton, PharmD7, Eilish McCann, PhD6. P0392 - Dupilumab Improves Dysphagia Symptoms in Adolescent and Adult Patients With Eosinophilic Esophagitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Evan S. Dellon, MD, MPH1, Marc E. Rothenberg, MD, PhD2, Albert J. Bredenoord, MD3, Ikuo Hirano, MD4, Mirna Chehade, MD, MPH5, Zhen Chen, PhD, MPH, MS, MBA6, Juby A. Jacob-Nara, MD, DHSc, MPH, MBA7, Amr Radwan, MBBCh, MA6, Sarette T. Tilton, PharmD7, Eilish McCann, PhD6
1University of North Carolina School of Medicine, Chapel Hill, NC; 2Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH; 3Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 4Feinberg School of Medicine, Northwestern University, Chicago, IL; 5Mount Sinai Center for Eosinophilic Disorders, Icahn School of Medicine at Mount Sinai, New York, NY; 6Regeneron Pharmaceuticals Inc., Tarrytown, NY; 7Sanofi, Bridgewater, NJ
Introduction: Dysphagia is the most common symptom of eosinophilic esophagitis (EoE) in adolescents and adults, but limited data exist regarding the thresholds for clinically relevant symptom response.
In the three-part phase 3 LIBERTY EoE TREET study (NCT03633617), dupilumab 300 mg weekly (qw) an antibody against the shared receptor component for interleukin IL-4 and IL-13, histologic, symptomatic, endoscopic, and molecular aspects of disease at 24 weeks versus placebo, with effects sustained up to 52 weeks in adult and adolescent patients with EoE. We aimed to evaluate the proportion of patients with EoE in Parts A and B of the LIBERTY EoE TREET study achieving a range of response thresholds in the Dysphagia Symptom Questionnaire (DSQ) biweekly total score, a validated patient-reported outcome.
Methods: Patients were randomized 1:1 to receive dupilumab 300 mg qw or placebo (Part A, n=42/39; Part B, n=80/79). For this secondary analysis, endpoints were proportion of patients achieving a ≥25%, ≥50%, ≥75%, or =100% reduction in DSQ total score at Week 24, calculated based on daily questionnaire responses over the 14-days prior to Week 24. Responders at each threshold were compared between dupilumab and placebo.
Results: The proportions of patients achieving a ≥25%, ≥50%, ≥75%, or =100% reduction from BL in DSQ total score for dupilumab versus placebo at Week 24 were:
Part A: 76%/44%; 71%/31%; 62%/23%; and 33%/13% (all P< 0.05 versus placebo). Part B: 78%/61%; 69%/43%; 55%/28%; and 29%/8% (all P< 0.05 versus placebo; Figure)
Discussion: A significantly greater proportion of dupilumab-treated patients had a reduction in DSQ score versus placebo at 24 weeks. Of these levels, it remains to be determined what DSQ response threshold optimally correlates with histologic, endoscopic, and molecular responses.

Figure: Figure: Proportion of Patients Achieving a ≥25%, ≥50%, ≥75%, or ≥100% Reduction in DSQ Total Score at Week 24
*P<0.05
P-values were derived by Cochran-Mantel-Haenszel test stratified by age group (≥12 to <18 vs ≥18) and use of PPI at randomization (Yes vs No).
CI, confidence interval; DSQ, Dysphagia Symptom Questionnaire; PPI, proton pump inhibitor; qw, once weekly.
*P<0.05
P-values were derived by Cochran-Mantel-Haenszel test stratified by age group (≥12 to <18 vs ≥18) and use of PPI at randomization (Yes vs No).
CI, confidence interval; DSQ, Dysphagia Symptom Questionnaire; PPI, proton pump inhibitor; qw, once weekly.
Disclosures:
Evan Dellon: Abbott – Consultant. Abbvie – Consultant. Adare/Ellodi – Consultant, Grant/Research Support. Aimmune – Consultant. Akesobio – Consultant. Alfasigma – Consultant. ALK – Consultant. Allakos – Consultant, Grant/Research Support. Amgen – Consultant. Aqilion – Consultant. Arena/Pfizer – Consultant, Grant/Research Support. Aslan – Consultant. AstraZeneca – Consultant, Grant/Research Support. Avir – Consultant. Banner Pharmaceuticals – Grant/Research Support. Biorasi – Consultant. Calypso – Consultant. Celgene/Receptos/BMS – Consultant, Grant/Research Support. Celldex – Consultant. Eli Lilly – Consultant. EsoCap – Consultant. Eupraxia – Consultant. Ferring – Consultant. Gossamer Bio – Consultant. GSK – Consultant, Grant/Research Support. Holoclara – Consultant, Grant/Research Support. Invea – Consultant, Grant/Research Support. Knightpoint – Consultant. Landos – Consultant. LucidDx – Consultant. Meritage – Grant/Research Support. Miraca – Grant/Research Support. Morphic – Consultant. Nexstone Immunology – Consultant. Nutricia – Consultant, Grant/Research Support. Parexel/Calyx – Consultant. Phathom – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Revolo Biotherapeutics – Consultant, Grant/Research Support. Robarts/Alimentiv – Consultant. Salix – Consultant. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Upstream Bio – Consultant.
Marc Rothenberg: Allakos – Consultant. AstraZeneca – Consultant. BMS – Consultant. Celldex – Consultant, Equity interest. ClostraBio – Consultant, Equity interest. Ellodi Pharmaceuticals – Consultant. GSK – Consultant. Guidepoint – Consultant. Mapi Research Trust – Royalties. Nextstone One – Consultant, Equity interest. PulmOne – Consultant, Equity interest. Regeneron Pharmaceuticals Inc. – Consultant. Revolo – Consultant. Sanofi – Consultant. Santa Ana Bio – Consultant, Equity interest. Serpin Pharma – Consultant, Equity interest. Spoon Guru – Consultant, Equity interest. Teva Pharmaceuticals – Royalties. UpToDate – Royalties.
Albert Bredenoord: Alimentiv – Consultant, Speakers Bureau. Aqilion – Consultant, Speakers Bureau. AstraZeneca – Consultant. Dr. Falk Pharma – Consultant, Grant/Research Support. Eupraxia – Consultant, Speakers Bureau. Laborie – Consultant. Medtronic – Consultant. Norgine – Grant/Research Support. Nutricia – Grant/Research Support. Reckitt – Consultant, Speakers Bureau. Regeneron Pharmaceuticals Inc. – Consultant. Sanofi – Consultant. SST – Grant/Research Support. Thelial – Grant/Research Support.
Ikuo Hirano: Adare/Ellodi – Consultant, Grant/Research Support. Allakos – Consultant, Grant/Research Support. Amgen – Consultant. Arena/Pfizer – Consultant, Grant/Research Support. Aslan – Consultant. AstraZeneca – Consultant, Grant/Research Support. Celgene/Receptos/Bristol-Myers Squibb – Consultant, Grant/Research Support. Celldex – Consultant. EsoCap – Consultant. Gossamer Bio – Consultant. Meritage – Grant/Research Support. Nexstone Immunology – Consultant. NIH – Grant/Research Support. Parexel/Calyx – Consultant. Phathom – Consultant. Regeneron – Consultant, Grant/Research Support. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support.
Mirna Chehade: Adare/Ellodi – Consultant, Grant/Research Support. Allakos – Consultant, Grant/Research Support. AstraZeneca – Consultant, Grant/Research Support. BMS – Consultant. Danone – Grant/Research Support. Nexstone Immunology – Consultant. Phathom – Consultant. Recludix – Consultant. Regeneron Pharmaceuticals Inc. – Consultant, Grant/Research Support. Sanofi – Consultant. Shire/Takeda – Consultant, Grant/Research Support.
Zhen Chen: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Juby Jacob-Nara: Sanofi – Employee, Stock Options.
Amr Radwan: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Sarette Tilton: Sanofi – Employee, Stock Options.
Eilish McCann: Regeneron Pharmaceuticals Inc. – Employee, Shareholder.
Evan S. Dellon, MD, MPH1, Marc E. Rothenberg, MD, PhD2, Albert J. Bredenoord, MD3, Ikuo Hirano, MD4, Mirna Chehade, MD, MPH5, Zhen Chen, PhD, MPH, MS, MBA6, Juby A. Jacob-Nara, MD, DHSc, MPH, MBA7, Amr Radwan, MBBCh, MA6, Sarette T. Tilton, PharmD7, Eilish McCann, PhD6. P0392 - Dupilumab Improves Dysphagia Symptoms in Adolescent and Adult Patients With Eosinophilic Esophagitis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

