Sunday Poster Session
Category: General Endoscopy
P0551 - Risk of Diabetic Ketoacidosis Post-Colonoscopy in Patients With Type 2 DM Taking Sodium-Glucose Co-Transporter-2 Inhibitors
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Julton Tomanguillo Chumbe, MD
Charleston Area Medical Center, West Virginia State University Charleston Division
Charleston, WV
Presenting Author(s)
Julton Tomanguillo Chumbe, MD, Frank Annie, PhD, Mark Ayoub, MD, Pariza Aijaz, MD, Medha Rajamanuri, MD, Vishnu Naravadi, MD
Charleston Area Medical Center, West Virginia State University Charleston Division, Charleston, WV
Introduction: Patients using Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2i) are at risk of rare but potentially life-threatening complication of euglycemic diabetic ketoacidosis (DKA) precipitated by fasting state, diet modifications, surgical procedures, and inappropriate management in perioperative period. No studies exist assessing this risk in patients undergoing colonoscopy. This study aims to assess the risk and rates of DKA among SGLT-2i users who underwent a colonoscopy.
Methods: Adult patients with type 2 diabetes mellitus (T2DM) between 45- and 75-years old undergoing colonoscopy between January 2007 and March 2023 were identified using TriNetX - a de-identified database of healthcare organizations from four different countries. Patients were divided into two cohorts; patients who were on SGLT-2i at least 1 week prior to colonoscopy and patients not taking SGLT-2i. The following SGLT-2i medications were evaluated: empagliflozin, canagliflozin, ertugliflozin, and dapagliflozin. We compared the rate of DKA up to 3 days post-colonoscopy, severity of DKA, and mortality between propensity matched (PSM) pairs of patients. Arterial pH and/or serum bicarbonate levels were used to further stratify the severity of DKA.
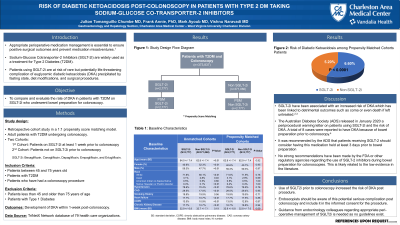
Results: A total of 373,837 patients were included in the analysis. Of those, 0.74% (n=2,777) were taking SGLT-2i at least 1 week prior to colonoscopy, and 99.26% (n=371,060) were not on SGLT-2i. The pre-PSM data showed that patients on SGLT-2i were younger and had a higher rate of key comorbidities (Table 1). Two PSM cohorts were created using 1:1 mode (2,777/2,777). The patients on SGLT-2i had a significantly higher rate of DKA (6.6% vs 5.2%, P=0.034) when compared to patients not on SGLT-2i. The severity of DKA did not differ among the two groups - mild-DKA (0.9% vs 1.2%, p= 0.30), moderated-DKA (0.43% vs 0.36%, P=0.66); severe-DKA (0.36 vs 0.26, P=1.00). The 14-day overall mortality was similar between both groups (0.36% vs 0.36%, P=1.00) and was confirmed with a Kaplan Meier estimator at 14 days with a log-rank of (P< 0.046).
Discussion: The use of SGLT-2i at least a week prior to colonoscopy significantly increased the risk of DKA post procedure. Endoscopists should be aware of this potential serious complication post colonoscopy and seek guidance from our endocrinology counterparts. Guidance from American societies about the appropriate perioperative management of patients using these medications is awaited.
Disclosures:
Julton Tomanguillo Chumbe, MD, Frank Annie, PhD, Mark Ayoub, MD, Pariza Aijaz, MD, Medha Rajamanuri, MD, Vishnu Naravadi, MD. P0551 - Risk of Diabetic Ketoacidosis Post-Colonoscopy in Patients With Type 2 DM Taking Sodium-Glucose Co-Transporter-2 Inhibitors, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Charleston Area Medical Center, West Virginia State University Charleston Division, Charleston, WV
Introduction: Patients using Sodium-Glucose Cotransporter-2 Inhibitors (SGLT-2i) are at risk of rare but potentially life-threatening complication of euglycemic diabetic ketoacidosis (DKA) precipitated by fasting state, diet modifications, surgical procedures, and inappropriate management in perioperative period. No studies exist assessing this risk in patients undergoing colonoscopy. This study aims to assess the risk and rates of DKA among SGLT-2i users who underwent a colonoscopy.
Methods: Adult patients with type 2 diabetes mellitus (T2DM) between 45- and 75-years old undergoing colonoscopy between January 2007 and March 2023 were identified using TriNetX - a de-identified database of healthcare organizations from four different countries. Patients were divided into two cohorts; patients who were on SGLT-2i at least 1 week prior to colonoscopy and patients not taking SGLT-2i. The following SGLT-2i medications were evaluated: empagliflozin, canagliflozin, ertugliflozin, and dapagliflozin. We compared the rate of DKA up to 3 days post-colonoscopy, severity of DKA, and mortality between propensity matched (PSM) pairs of patients. Arterial pH and/or serum bicarbonate levels were used to further stratify the severity of DKA.
Results: A total of 373,837 patients were included in the analysis. Of those, 0.74% (n=2,777) were taking SGLT-2i at least 1 week prior to colonoscopy, and 99.26% (n=371,060) were not on SGLT-2i. The pre-PSM data showed that patients on SGLT-2i were younger and had a higher rate of key comorbidities (Table 1). Two PSM cohorts were created using 1:1 mode (2,777/2,777). The patients on SGLT-2i had a significantly higher rate of DKA (6.6% vs 5.2%, P=0.034) when compared to patients not on SGLT-2i. The severity of DKA did not differ among the two groups - mild-DKA (0.9% vs 1.2%, p= 0.30), moderated-DKA (0.43% vs 0.36%, P=0.66); severe-DKA (0.36 vs 0.26, P=1.00). The 14-day overall mortality was similar between both groups (0.36% vs 0.36%, P=1.00) and was confirmed with a Kaplan Meier estimator at 14 days with a log-rank of (P< 0.046).
Discussion: The use of SGLT-2i at least a week prior to colonoscopy significantly increased the risk of DKA post procedure. Endoscopists should be aware of this potential serious complication post colonoscopy and seek guidance from our endocrinology counterparts. Guidance from American societies about the appropriate perioperative management of patients using these medications is awaited.
Disclosures:
Julton Tomanguillo Chumbe indicated no relevant financial relationships.
Frank Annie indicated no relevant financial relationships.
Mark Ayoub indicated no relevant financial relationships.
Pariza Aijaz indicated no relevant financial relationships.
Medha Rajamanuri indicated no relevant financial relationships.
Vishnu Naravadi indicated no relevant financial relationships.
Julton Tomanguillo Chumbe, MD, Frank Annie, PhD, Mark Ayoub, MD, Pariza Aijaz, MD, Medha Rajamanuri, MD, Vishnu Naravadi, MD. P0551 - Risk of Diabetic Ketoacidosis Post-Colonoscopy in Patients With Type 2 DM Taking Sodium-Glucose Co-Transporter-2 Inhibitors, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
