Sunday Poster Session
Category: GI Bleeding
P0600 - Risk of Bleeding During Percutaneous Endoscopic Gastrostomy Tube Insertion in Patients on Continued Antiplatelet Therapy: A Systematic Review and Meta-analysis
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Harsimran K. Bhatia, MBBS
University of Texas Health Science Center at Houston
Houston, TX
Presenting Author(s)
Award: Presidential Poster Award
Harsimran K. Bhatia, MBBS1, Nirav Thosani, MD2
1University of Texas Health Science Center at Houston, Houston, TX; 2McGovern Medical School at UTHealth, Houston, TX
Introduction: Percutaneous endoscopic gastrostomy (PEG) tube insertion is commonly performed for providing nutritional support for patients with dysphagia. Many patients are concurrently on single or dual antiplatelet therapy to prevent adverse cardiovascular events. Although current guidelines recommend interruption of antiplatelets prior to PEG tube insertion, the risk-benefit ratio is unknown. This updated systematic review aims to assess the bleeding risk associated with antiplatelet therapy during PEG placement.
Methods: We performed a search of Embase, Medline, and Web of Science databases to identify studies investigating the risk of bleeding among patients undergoing PEG placement while on uninterrupted antiplatelet therapy. Uninterrupted antiplatelet therapy was defined as use of aspirin ≤1 day and/or P2Y12 inhibitors ≤5 days of PEG tube placement. The primary outcome of interest was the occurrence of any bleeding events following PEG insertion. Pooled incidence of post-PEG tube bleeding, including 95% confidence intervals (CI) were calculated. We also calculated pooled risk ratios (RR) using a random-effects model. Heterogeneity among the studies was assessed using the Higgins I2 value.
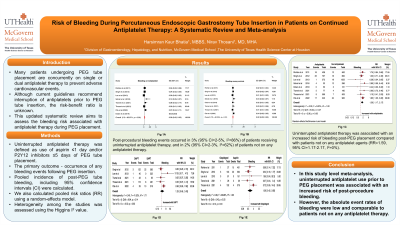
Results: Out of 1236 initial citations, eight observational studies with a total of 6939 patients were included. Up to 35% of the total population underwent PEG insertion while receiving uninterrupted single or dual antiplatelet therapy. The mean age was 64-72 years and 45% of the patients were females. Post-procedural bleeding events occurred in 3% (95% CI=2-5%, I2=86%) of patients receiving uninterrupted antiplatelet therapy, and in 2% (95% CI=2-3%, I2=62%) of patients not on any antiplatelet therapy. Uninterrupted antiplatelet therapy was associated with an increased risk of bleeding post-PEG placement compared with patients not on any antiplatelet agents (RR=1.59, 95% CI=1.17-2.17, I2=0%). However, there was no significant difference in the risk of bleeding among patients undergoing PEG placement while on uninterrupted clopidogrel monotherapy compared with aspirin monotherapy.
Discussion: In this study level meta-analysis, uninterrupted antiplatelet use prior to PEG placement was associated with an increased risk of post-procedure bleeding. However, the absolute event rates of bleeding were low and comparable to patients not on any antiplatelet therapy.

Disclosures:
Harsimran K. Bhatia, MBBS1, Nirav Thosani, MD2. P0600 - Risk of Bleeding During Percutaneous Endoscopic Gastrostomy Tube Insertion in Patients on Continued Antiplatelet Therapy: A Systematic Review and Meta-analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Harsimran K. Bhatia, MBBS1, Nirav Thosani, MD2
1University of Texas Health Science Center at Houston, Houston, TX; 2McGovern Medical School at UTHealth, Houston, TX
Introduction: Percutaneous endoscopic gastrostomy (PEG) tube insertion is commonly performed for providing nutritional support for patients with dysphagia. Many patients are concurrently on single or dual antiplatelet therapy to prevent adverse cardiovascular events. Although current guidelines recommend interruption of antiplatelets prior to PEG tube insertion, the risk-benefit ratio is unknown. This updated systematic review aims to assess the bleeding risk associated with antiplatelet therapy during PEG placement.
Methods: We performed a search of Embase, Medline, and Web of Science databases to identify studies investigating the risk of bleeding among patients undergoing PEG placement while on uninterrupted antiplatelet therapy. Uninterrupted antiplatelet therapy was defined as use of aspirin ≤1 day and/or P2Y12 inhibitors ≤5 days of PEG tube placement. The primary outcome of interest was the occurrence of any bleeding events following PEG insertion. Pooled incidence of post-PEG tube bleeding, including 95% confidence intervals (CI) were calculated. We also calculated pooled risk ratios (RR) using a random-effects model. Heterogeneity among the studies was assessed using the Higgins I2 value.
Results: Out of 1236 initial citations, eight observational studies with a total of 6939 patients were included. Up to 35% of the total population underwent PEG insertion while receiving uninterrupted single or dual antiplatelet therapy. The mean age was 64-72 years and 45% of the patients were females. Post-procedural bleeding events occurred in 3% (95% CI=2-5%, I2=86%) of patients receiving uninterrupted antiplatelet therapy, and in 2% (95% CI=2-3%, I2=62%) of patients not on any antiplatelet therapy. Uninterrupted antiplatelet therapy was associated with an increased risk of bleeding post-PEG placement compared with patients not on any antiplatelet agents (RR=1.59, 95% CI=1.17-2.17, I2=0%). However, there was no significant difference in the risk of bleeding among patients undergoing PEG placement while on uninterrupted clopidogrel monotherapy compared with aspirin monotherapy.
Discussion: In this study level meta-analysis, uninterrupted antiplatelet use prior to PEG placement was associated with an increased risk of post-procedure bleeding. However, the absolute event rates of bleeding were low and comparable to patients not on any antiplatelet therapy.

Figure: Fig (A). Pooled incidence of post PEG tube bleeding events in patients on antiplatelet therapy,
Fig (B). Pooled incidence of post PEG tube bleeding events in control group not on antiplatelet therapy,
Fig (C). Pooled analysis for the relative risk of any PEG-related bleeding event in patients under antiplatelet therapy compared to controls not receiving antiplatelet agents,
Fig (D). Pooled analysis for the relative risk of any PEG-related bleeding event in patients under dual antiplatelet therapy compared to patients on single antiplatelet therapy.
Fig (B). Pooled incidence of post PEG tube bleeding events in control group not on antiplatelet therapy,
Fig (C). Pooled analysis for the relative risk of any PEG-related bleeding event in patients under antiplatelet therapy compared to controls not receiving antiplatelet agents,
Fig (D). Pooled analysis for the relative risk of any PEG-related bleeding event in patients under dual antiplatelet therapy compared to patients on single antiplatelet therapy.
Disclosures:
Harsimran Bhatia indicated no relevant financial relationships.
Nirav Thosani: Abbvie – Consultant. Boston Scientific Corp – Consultant. Pentax America – Consultant. ROSEAID inc – Creatorship rights.
Harsimran K. Bhatia, MBBS1, Nirav Thosani, MD2. P0600 - Risk of Bleeding During Percutaneous Endoscopic Gastrostomy Tube Insertion in Patients on Continued Antiplatelet Therapy: A Systematic Review and Meta-analysis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

