Sunday Poster Session
Category: IBD
P0730 - Guselkumab Improves Abdominal Pain and Bowel Urgency Symptoms in Patients With Moderately to Severely Active Ulcerative Colitis: Results From the Phase 3 QUASAR Induction Study
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

David Rubin, MD, FACG
University of Chicago Medicine, Inflammatory Bowel Disease Center
Chicago, IL
Presenting Author(s)
David T. Rubin, MD, FACG1, Julian Panés, MD, PhD2, Brian G. Feagan, MD3, Shadi Yarandi, MD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Chenglong Han, MD, PhD5, Ye Miao, MS4, Hongyan Zhang, PhD4, Yun Qiu, MD, PhD6, Maria Klopocka, MD7, George Duvall, MD8, Gary R. Lichtenstein, MD9, Tadakazu Hisamatsu, MD, PhD10, Brian Bressler, MD, MS, FRCPC11, Laurent Peyrin-Biroulet, MD, PhD12
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 3Western University, London, ON, Canada; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Global Services, LLC, Malvern, PA; 6The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China; 7Szpital Uniwersytecki nr 2 im. dr. Jana Biziela w Bydgoszczy, Bydgoszcz, Pomorskie, Poland; 8Tyler Research Institute, LLC, Tyler, TX; 9University of Pennsylvania, Philadelphia, PA; 10Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 11St. Paul’s Hospital, Vancouver, BC, Canada; 12Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France
Introduction: Abdominal pain and bowel urgency are prevalent, burdensome symptoms experienced by patients (pts) with ulcerative colitis (UC) that impact daily life, including social activities. In this study, we use data from the QUASAR Phase 3 Induction Study to assess efficacy of guselkumab (GUS) on abdominal pain, bowel urgency, and the impact of bowel urgency on pts’ lives.
Methods: Pts were randomized 3:2 to receive IV GUS 200mg or placebo (PBO) at Weeks (Wks) 0, 4, and 8. Abdominal pain and bowel urgency were evaluated at baseline and Wk12 using items from the Inflammatory Bowel Disease Questionnaire, where pts rated trouble with abdominal pain, symptoms of bowel urgency, and the impact of bowel urgency over the past 2 wks using 7-point scales (1=all of the time to 7=none of the time). A ≥2 point increase from baseline was considered clinically meaningful improvement. All analyses were prespecified but not multiplicity controlled; all p values are nominal.
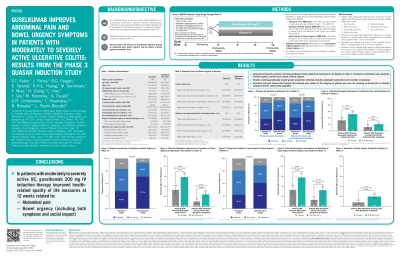
Results: At baseline, percentages of pts with abdominal pain (GUS 77.7% and PBO 77.9%), symptoms of bowel urgency (GUS 86.0% and PBO 83.2%), and impact of bowel urgency (GUS 70.8% and PBO 70.4%) at least a little of the time (score ≤5) were similar between groups. GUS-treated pts showed greater improvements at Wk12 in these outcomes compared with PBO (Table 1). For abdominal pain, 52.0% of GUS-treated pts had clinically meaningful improvements from baseline at Wk12 vs 33.0% in the PBO group (p< 0.001), and 21.1% vs 12.3%, respectively, of those with abdominal pain at baseline had resolution at Wk12 (p=0.004; Fig. 1A). For symptoms of bowel urgency, 58.6% vs 33.0%, respectively, had clinically meaningful improvements (p< 0.001; Fig. 1B), and 24.0% vs 9.8%, respectively, of those with symptoms of bowel urgency at baseline had resolution at Wk12 (p< 0.001). Similarly, 57.7% vs 33.0%, respectively, had clinically meaningful improvements in the impact of bowel urgency (p< 0.001; Fig. 1B), and 32.4% vs 13.1%, respectively, of those impacted at baseline had resolution at Wk12 (p< 0.001). Symptoms and impact of bowel urgency were combined into a bowel urgency score; of pts with symptoms or impact scores ≤6 at baseline, 19.7% versus 8.2%, respectively, had resolution of both at Wk12 (p< 0.001; Fig. 1B).
Discussion: Pts receiving GUS showed clinically meaningful improvements in health-related quality of life measures related to abdominal pain and bowel urgency, including both the symptoms of bowel urgency and impact on pts’ lives.

Disclosures:
David T. Rubin, MD, FACG1, Julian Panés, MD, PhD2, Brian G. Feagan, MD3, Shadi Yarandi, MD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Chenglong Han, MD, PhD5, Ye Miao, MS4, Hongyan Zhang, PhD4, Yun Qiu, MD, PhD6, Maria Klopocka, MD7, George Duvall, MD8, Gary R. Lichtenstein, MD9, Tadakazu Hisamatsu, MD, PhD10, Brian Bressler, MD, MS, FRCPC11, Laurent Peyrin-Biroulet, MD, PhD12. P0730 - Guselkumab Improves Abdominal Pain and Bowel Urgency Symptoms in Patients With Moderately to Severely Active Ulcerative Colitis: Results From the Phase 3 QUASAR Induction Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 3Western University, London, ON, Canada; 4Janssen Research & Development, LLC, Spring House, PA; 5Janssen Global Services, LLC, Malvern, PA; 6The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China; 7Szpital Uniwersytecki nr 2 im. dr. Jana Biziela w Bydgoszczy, Bydgoszcz, Pomorskie, Poland; 8Tyler Research Institute, LLC, Tyler, TX; 9University of Pennsylvania, Philadelphia, PA; 10Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 11St. Paul’s Hospital, Vancouver, BC, Canada; 12Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France
Introduction: Abdominal pain and bowel urgency are prevalent, burdensome symptoms experienced by patients (pts) with ulcerative colitis (UC) that impact daily life, including social activities. In this study, we use data from the QUASAR Phase 3 Induction Study to assess efficacy of guselkumab (GUS) on abdominal pain, bowel urgency, and the impact of bowel urgency on pts’ lives.
Methods: Pts were randomized 3:2 to receive IV GUS 200mg or placebo (PBO) at Weeks (Wks) 0, 4, and 8. Abdominal pain and bowel urgency were evaluated at baseline and Wk12 using items from the Inflammatory Bowel Disease Questionnaire, where pts rated trouble with abdominal pain, symptoms of bowel urgency, and the impact of bowel urgency over the past 2 wks using 7-point scales (1=all of the time to 7=none of the time). A ≥2 point increase from baseline was considered clinically meaningful improvement. All analyses were prespecified but not multiplicity controlled; all p values are nominal.
Results: At baseline, percentages of pts with abdominal pain (GUS 77.7% and PBO 77.9%), symptoms of bowel urgency (GUS 86.0% and PBO 83.2%), and impact of bowel urgency (GUS 70.8% and PBO 70.4%) at least a little of the time (score ≤5) were similar between groups. GUS-treated pts showed greater improvements at Wk12 in these outcomes compared with PBO (Table 1). For abdominal pain, 52.0% of GUS-treated pts had clinically meaningful improvements from baseline at Wk12 vs 33.0% in the PBO group (p< 0.001), and 21.1% vs 12.3%, respectively, of those with abdominal pain at baseline had resolution at Wk12 (p=0.004; Fig. 1A). For symptoms of bowel urgency, 58.6% vs 33.0%, respectively, had clinically meaningful improvements (p< 0.001; Fig. 1B), and 24.0% vs 9.8%, respectively, of those with symptoms of bowel urgency at baseline had resolution at Wk12 (p< 0.001). Similarly, 57.7% vs 33.0%, respectively, had clinically meaningful improvements in the impact of bowel urgency (p< 0.001; Fig. 1B), and 32.4% vs 13.1%, respectively, of those impacted at baseline had resolution at Wk12 (p< 0.001). Symptoms and impact of bowel urgency were combined into a bowel urgency score; of pts with symptoms or impact scores ≤6 at baseline, 19.7% versus 8.2%, respectively, had resolution of both at Wk12 (p< 0.001; Fig. 1B).
Discussion: Pts receiving GUS showed clinically meaningful improvements in health-related quality of life measures related to abdominal pain and bowel urgency, including both the symptoms of bowel urgency and impact on pts’ lives.

Figure: Figure 1. Percentages of patients with clinically meaningful improvement (≥2 point change) from baseline to Wk12 among those with symptoms at baseline at least a little bit of the time (score ≤5) and resolution of symptoms at Wk12 among those with symptoms at baseline hardly any of the time (score ≤6) for symptoms of (A) abdominal pain and (B) bowel urgency. All p-values presented are nominal.
| Outcome | Change from baseline | Placebo IV (N=261) | Guselkumab 200 mg IV (N=405) | P-value |
| Abdominal pain | Improved | 123 (47.1%) | 267 (65.9%) | < 0.001 |
| No change | 102 (39.1%) | 103 (25.4%) | ||
| Worsened | 36 (13.8%) | 35 (8.6%) | ||
| Symptoms of bowel urgency | Improved | 125 (47.9%) | 288 (71.1%) | < 0.001 |
| No change | 93 (35.6%) | 89 (22.0%) | ||
| Worsened | 43 (16.5%) | 28 (6.9%) | ||
| Impact of bowel urgency | Improved | 110 (42.1%) | 246 (60.7%) | < 0.001 |
| No change | 101 (38.7%) | 115 (28.4%) | ||
| Worsened | 50 (19.2%) | 44 (10.9%) | ||
| All p-values are nominal. P-values were based on the Cochran-Mantel-Haenszel (CMH) chi-square Row Mean Score test, stratified by ADT-failure status (Yes/No) and concomitant use of corticosteroids at baseline (Yes/No). | ||||
Table: Table 1. Changes from baseline to Wk12 in abdominal pain, symptoms of bowel urgency, and impact of bowel urgency
Disclosures:
David T. Rubin: AbbVie – Consultant. Alike Health – Stock Options. AltruBio – Consultant, Stock Options. Aslan Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. Chronicles – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Advisory Committee/Board Member. Crohn's & Colitis Foundation – Advisory Committee/Board Member. Datos Health – Stock Options. EcoR1 – Consultant. Eli Lilly – Consultant. GastroIntestinal Research Foundation – Grant/Research Support. Genentech/Roche – Consultant. Gilead Sciences – Consultant. Helmsley Charitable Trust – Grant/Research Support. Iterative Health – Consultant. Iterative Health – Stock Options. Janssen – Consultant. Kaleido Biosciences – Consultant. Pfizer – Consultant. Prometheus Biosciences – Consultant. Reistone – Consultant. Seres Therapeutics – Consultant. Syneos – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant. Trellus Health – Consultant.
Julian Panés: AbbVie – Grant/Research Support, Personal fees. Arena – Consultant. Athos – Consultant. Atomwise – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant. Genentech/Roche – Consultant. GlaxoSmithKline – Consultant. Immunic – Personal fees. Janssen – Consultant, payment for development of educational presentations. Mirum – Consultant. Morphic – Consultant. Origo – Consultant. Pandion – Consultant. Pfizer Inc – Grant/Research Support, payment for development of educational presentations. Progenity – Consultant. Revolo – Consultant. Takeda – payment for development of educational presentations. Theravance – Consultant. Wasserman – Consultant.
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Consultant. Applied Molecular Transport Inc. – Consultant. Arena Pharma – Consultant. Avir – Consultant. Azora Therapeutics – Consultant. Baxter – Consultant. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boston Pharma – Consultant. Bristol Myers Squibb – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Connect BioPharma – Consultant. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 – Advisory Committee/Board Member, Consultant. Eli Lilly – Consultant. Equillium – Consultant. Ermium – Consultant. Everest Clinical Research Corp. – Consultant. Ferring Pharmaceuticals – Consultant. First Wave – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Glenmark – Consultant. Gossamer Pharma – Consultant, stock shareholder. Hoffmann-LaRoche – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. ImmunExt – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Advisory Committee/Board Member, Consultant. Intact Therapeutics – Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Consultant. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. Novartis – Advisory Committee/Board Member. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Otsuka – Consultant. Pandion Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Therapeutics and Diagnostics – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. RedHill Biopharma – Consultant. Redx – Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Theravance – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. UCB Pharma – Consultant. VHSquared Ltd. – Consultant. Viatris – Consultant. Western University, Alimentiv Inc – Employee. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Shadi Yarandi: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Kuan-Hsiang G. Huang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Chenglong Han: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ye Miao: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Yun Qiu: Janssen – Consultant, clinical investigator.
Maria Klopocka: Janssen – Consultant, clinical investigator.
George Duvall: Janssen – Consultant, clinical investigator.
Gary R. Lichtenstein: Abbvie – Consultant. American College of Gastroenterology – Honorarium for Associate Editor of American Journal of Gastroenterology. American Gastroenterological Association – CME. American Regent – Consultant, Honorarium [CME Program]. Celgene – Consultant, Grant/Research Support. CellCeutrix – Consultant. Chemed – CME. Eli Lilly – Consultant, Data Safety Monitoring Board. Endo Pharmaceuticals – Consultant. Ferring – Consultant. Gastroenterology and Hepatology – Gastro-Hep Communication, Editor-Honorarium. Gilead – Consultant. IMEDEX – CME. Ironwood – CME. Janssen/ Janssen Orthobiotech – Consultant, Grant/Research Support, Funding to University of PA [IBD Fellow Education]. MedEd Consultants – Consultant. Merck – Consultant, Honorarium [CME Program]. Morphic Therapeutics – Consultant. Pfizer Pharmaceuticals – Consultant, Funding to University of PA [IBD Fellow Education]. Professional Communications, Inc – Royalty for writing Textbook. Prometheus Laboratories, Inc – Consultant. Romark – Consultant, Honorarium for CME. Salix Pharmaceuticals/Valeant – Consultant. Sandoz – Consultant. Shire Pharmaceuticals – Consultant. SLACK, Inc – Book Royalty. Springer Science and Business Media – Editor [Honorarium]. Takeda – Consultant, Grant/Research Support, Funding to University of PA [IBD Fellow Education]. UCB – Consultant, Grant/Research Support. University of Kentucky – CME. Up-To-Date – Author [Honorarium]. Vindico – CME. Virgo – Consultant, Stock Options.
Tadakazu Hisamatsu: AbbVie GK – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Alfresa Pharma Corporation and EA Pharma Co., Ltd – Grant/Research Support. Daiichi-Sankyo – Grant/Research Support. EA Pharma Co, Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Eli Lilly – Consultant. Gilead Sciences – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Janssen Pharmaceutical K.K. – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. JIMRO Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. KISSEI PHARMACEUTICAL CO., LTD – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Kyorin Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mochida Pharmacuetical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nichi-Iko Pharmaceutical Co., Ltd – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nippon Kayaku Co., Ltd – Grant/Research Support. Pfizer Japan Inc. – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Takeda Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Zeria Pharmaceutical Co., Ltd – Grant/Research Support.
Brian Bressler: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Alimentiv – Advisory Committee/Board Member. Allergan – Advisory Committee/Board Member. Alvine – Grant/Research Support. Amgen – Advisory Committee/Board Member, Grant/Research Support. AMT – Advisory Committee/Board Member. Bausch Health – Advisory Committee/Board Member. Boehringer Ingelheim – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Celgene – Advisory Committee/Board Member, Grant/Research Support. Ferring – Advisory Committee/Board Member, Speakers Bureau. Fresenius Kabi – Advisory Committee/Board Member. Genentech – Advisory Committee/Board Member, Grant/Research Support. Gilead – Advisory Committee/Board Member. GlaxoSmithKline – Grant/Research Support. Iterative Scopes – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Merck – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Microbiome Insights – Advisory Committee/Board Member. Mylan – Advisory Committee/Board Member. Novartis – Advisory Committee/Board Member, Speakers Bureau. Pendopharm – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member, Speakers Bureau. Protagonist – Advisory Committee/Board Member. Qu Biologic – Grant/Research Support, Stock Options. Takeda – Advisory Committee/Board Member, Speakers Bureau.
Laurent Peyrin-Biroulet: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Allergan – personal fees. Alma – personal fees. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Applied Molecular Transport – personal fees. Arena – personal fees. Biogaran – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – personal fees. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. CTMA – Stock Options. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Enterome – personal fees. Enthera – personal fees. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Forward Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Fresenius – personal fees. Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – personal fees. H.A.C. Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Hikma – personal fees. Hospira/Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Index Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lycera – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mitsubishi – Advisory Committee/Board Member, Consultant, Speakers Bureau. MSD – Grant/Research Support, personal fees. Mylan – personal fees. Nestlé – personal fees. Norgine – Advisory Committee/Board Member, Consultant, Speakers Bureau. Oppilan Pharma – personal fees. OSE Immunotherapeutics – personal fees. Pfizer – personal fees. Pharmacosmos – personal fees. Roche – personal fees. Samsung Bioepis – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sterna – personal fees. Sublimity Therapeutics – personal fees. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Theravance – Advisory Committee/Board Member, Consultant, Speakers Bureau. Tillots – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vifor – Advisory Committee/Board Member, Consultant, Speakers Bureau.
David T. Rubin, MD, FACG1, Julian Panés, MD, PhD2, Brian G. Feagan, MD3, Shadi Yarandi, MD4, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Chenglong Han, MD, PhD5, Ye Miao, MS4, Hongyan Zhang, PhD4, Yun Qiu, MD, PhD6, Maria Klopocka, MD7, George Duvall, MD8, Gary R. Lichtenstein, MD9, Tadakazu Hisamatsu, MD, PhD10, Brian Bressler, MD, MS, FRCPC11, Laurent Peyrin-Biroulet, MD, PhD12. P0730 - Guselkumab Improves Abdominal Pain and Bowel Urgency Symptoms in Patients With Moderately to Severely Active Ulcerative Colitis: Results From the Phase 3 QUASAR Induction Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
