Sunday Poster Session
Category: IBD
P0741 - Risankizumab for the Treatment of Moderate to Severe Crohn’s Disease: A Systematic Review of Literature
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Yashwitha Sai Pulakurthi, MBBS
New York Medical College-Saint Michael's Medical Center
Newark, New Jersey
Presenting Author(s)
Yashwitha Sai Pulakurthi, MBBS1, Gowthami Sai Kogilathota Jagirdhar, MD2, Navya Sadum, MBBS3, Praneeth Reddy Keesari, MD4, Rewanth Katamreddy, MD5, Yatinder Bains, MD6
1New York Medical College-Saint Michael's Medical Center, Newark, NJ; 2Saint Michael's Medical Center, Newark, NJ; 3Mayo Clinic, Jacksonville, FL; 4Staten Island University Hospital, Staten Island, NY; 5Saint Michael’s Medical Center, Newark, NJ; 6Saint Michael's Medical Center, New York Medical College, Newark, NJ
Introduction: Anti IL-23 inhibitors (Ustekinumab, Risankizumab, Mirikizumab, Brazikumab, Guselkumab, Tildrakizumab), novel therapeutic targets have been well studied for Psoriasis and Psoriatic arthritis. Risankizumab, an inhibitor of the p19 subunit of IL-23 was recently approved by the FDA for the treatment of moderate to severe Crohn’s disease. We attempted to systematically review the existing literature on this novel monoclonal antibody.
Methods: We performed a systematic review of literature databases like Pubmed, Cochrane, Embase, clinical trials.gov and google scholar from January 2014 to May 2023. Data pertaining to drug dose, frequency, induction/maintenance information, clinical outcomes and adverse events were interpreted using descriptive analysis.
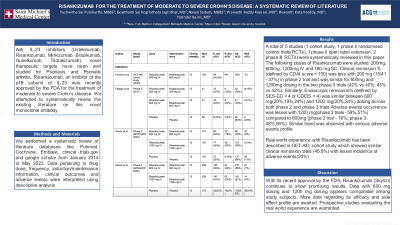
Results: A total of 5 studies (1 cohort study, 1 phase II randomised control trials(RCTs), 1 phase II open label extension, 2 phase III RCTs) were systematically reviewed in this paper. The following doses of Risankizumab were studied: 200mg, 600mg, 1200mg IV and 180 mg SC. Clinical remission % (defined by CDAI score < 150) was less with 200 mg (15/41 - 37%) in phase 2 trial and was similar for 600mg and 1200mg dosing in the two phase 3 trials (42% vs 40%; 45% vs 42%). Similarly, Endoscopic remission% (defined by SES-CD < 4 or CDEIS < 4) was similar between 600 mg(20%,19%,24%) and 1200 mg(20%,24%) dosing across both phase 2 and phase 3 trials.Adverse events occurrence was lesser with 1200 mg(phase 3 trials- 59%,51%) compared to 600mg (phase 2 trial - 76%, phase 3 48%,56%). Similar trend was observed with serious adverse events profile.
Real world experience with Risankizumab has been described in GET-AID cohort study which showed similar clinical remission rates (45.8%) with lesser incidence of adverse events(20%)
Discussion: With its recent approval by the FDA, Risankizumab (Skyrizi) continues to show promising results. Data with 600 mg dosing and 1200 mg dosing appears comparable among study subjects. More data regarding its efficacy and side effect profile are awaited. Prospective studies evaluating the real world experience are warranted.
Disclosures:
Yashwitha Sai Pulakurthi, MBBS1, Gowthami Sai Kogilathota Jagirdhar, MD2, Navya Sadum, MBBS3, Praneeth Reddy Keesari, MD4, Rewanth Katamreddy, MD5, Yatinder Bains, MD6. P0741 - Risankizumab for the Treatment of Moderate to Severe Crohn’s Disease: A Systematic Review of Literature, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1New York Medical College-Saint Michael's Medical Center, Newark, NJ; 2Saint Michael's Medical Center, Newark, NJ; 3Mayo Clinic, Jacksonville, FL; 4Staten Island University Hospital, Staten Island, NY; 5Saint Michael’s Medical Center, Newark, NJ; 6Saint Michael's Medical Center, New York Medical College, Newark, NJ
Introduction: Anti IL-23 inhibitors (Ustekinumab, Risankizumab, Mirikizumab, Brazikumab, Guselkumab, Tildrakizumab), novel therapeutic targets have been well studied for Psoriasis and Psoriatic arthritis. Risankizumab, an inhibitor of the p19 subunit of IL-23 was recently approved by the FDA for the treatment of moderate to severe Crohn’s disease. We attempted to systematically review the existing literature on this novel monoclonal antibody.
Methods: We performed a systematic review of literature databases like Pubmed, Cochrane, Embase, clinical trials.gov and google scholar from January 2014 to May 2023. Data pertaining to drug dose, frequency, induction/maintenance information, clinical outcomes and adverse events were interpreted using descriptive analysis.
Results: A total of 5 studies (1 cohort study, 1 phase II randomised control trials(RCTs), 1 phase II open label extension, 2 phase III RCTs) were systematically reviewed in this paper. The following doses of Risankizumab were studied: 200mg, 600mg, 1200mg IV and 180 mg SC. Clinical remission % (defined by CDAI score < 150) was less with 200 mg (15/41 - 37%) in phase 2 trial and was similar for 600mg and 1200mg dosing in the two phase 3 trials (42% vs 40%; 45% vs 42%). Similarly, Endoscopic remission% (defined by SES-CD < 4 or CDEIS < 4) was similar between 600 mg(20%,19%,24%) and 1200 mg(20%,24%) dosing across both phase 2 and phase 3 trials.Adverse events occurrence was lesser with 1200 mg(phase 3 trials- 59%,51%) compared to 600mg (phase 2 trial - 76%, phase 3 48%,56%). Similar trend was observed with serious adverse events profile.
Real world experience with Risankizumab has been described in GET-AID cohort study which showed similar clinical remission rates (45.8%) with lesser incidence of adverse events(20%)
Discussion: With its recent approval by the FDA, Risankizumab (Skyrizi) continues to show promising results. Data with 600 mg dosing and 1200 mg dosing appears comparable among study subjects. More data regarding its efficacy and side effect profile are awaited. Prospective studies evaluating the real world experience are warranted.
Table: Table showing characteristics and outcomes of included trials of Risankizumab
Disclosures:
Yashwitha Sai Pulakurthi indicated no relevant financial relationships.
Gowthami Sai Kogilathota Jagirdhar indicated no relevant financial relationships.
Navya Sadum indicated no relevant financial relationships.
Praneeth Reddy Keesari indicated no relevant financial relationships.
Rewanth Katamreddy indicated no relevant financial relationships.
Yatinder Bains indicated no relevant financial relationships.
Yashwitha Sai Pulakurthi, MBBS1, Gowthami Sai Kogilathota Jagirdhar, MD2, Navya Sadum, MBBS3, Praneeth Reddy Keesari, MD4, Rewanth Katamreddy, MD5, Yatinder Bains, MD6. P0741 - Risankizumab for the Treatment of Moderate to Severe Crohn’s Disease: A Systematic Review of Literature, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
