Sunday Poster Session
Category: IBD
P0745 - Real-World Effectiveness of Advanced Therapies on Bowel Urgency in Patients With Ulcerative Colitis in the United States: Results from SPARC IBD
- TH
Theresa Hunter Gibble, PhD, MPH
Eli Lilly and Company
Indianapolis, IN
Presenting Author(s)
1University of Pennsylvania, Philadelphia, PA; 2Eli Lilly and Company, Indianapolis, IN; 3Syneos Health, Morrisville, NC; 4TechData Service Company, Pennsylvania, PA
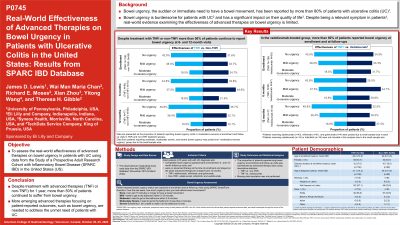
Introduction: Bowel urgency (BU), the sudden need for a bowel movement, affects over 80% of patients with ulcerative colitis (UC).1 However, real-world evidence examining the impact of different advanced therapies on BU in patients with UC is limited. We aimed to assess the prevalence of BU among patients with UC treated with advanced therapies.
Methods: This retrospective longitudinal study utilized data from the Study of a Prospective Adult Research Cohort with Inflammatory Bowel Disease (SPARC IBD) registry in the US from November 2016 to March 2022. Patients were required to be on advanced therapy at the time of enrollment and remain on the same advanced therapy for at least 6 or 12 months. Advanced therapies included TNFi (adalimumab, infliximab, and golimumab) and non-TNFi (ustekinumab, vedolizumab, and tofacitinib). Patient-reported outcomes, including BU questionnaire, were collected quarterly on a scale ranging from 0 (none) to 4 (severe). BU categories of mild, moderate, moderately severe, and severe were combined to form the group of patients who experienced BU. The proportion of patients who experienced BU at enrollment, 6-, and 12-month visits was summarized by advanced therapy class (TNFi versus non-TNFi) and by individual therapies using descriptive statistics.
Results: Of 203 patients (54.1% females; mean age: 42.4 years) included in this study, 108 received TNFi and 95 received non-TNFi at enrollment. BU was reported by 56.5% of TNFi patients and 68.4% of non-TNFi patients at enrollment. Despite treatment with advanced therapy for 12 months, >50% of patients continued to experience BU in the TNFi (53.2%) and non-TNFi (64.3%) groups (Table).
Compared to enrollment visit, the proportion of patients who reported BU at 12-month visit was comparable with TNFi therapy (56.5% vs 53.2%) and with vedolizumab (65.1% vs 67.5%; Figure).
Discussion: Patients with UC continue to experience bowel urgency despite treatment with advanced therapies for 1 year. Given that patients with UC perceive bowel urgency as the most relevant symptom2, better treatment options are needed.
References:
1. Nóbrega VG et al., Arq Gastroenterol. 2018;55(3):290-295.
2. Dubinsky MC et al. CC360, 2022;5(1):otac044.

* Data from patients who were receiving ustekinumab or tofacitinib for UC are not shown due to the small sample size.
TNFi: Tumor necrosis factor inhibitors (adalimumab, golimumab, and infliximab); Non-TNFi: Non-Tumor necrosis factor inhibitors (ustekinumab, vedolizumab, and tofacitinib).
Cohort | TNFi | Non-TNFi | All |
Enrollment | N=108 | N=95 | N=203 |
No urgency, n (%) | 47 (43.5) | 30 (31.6) | 77 (37.9) |
Urgency, n (%) | 61 (56.5) | 65 (68.4) | 126 (62.1) |
6 months | N=80 | N=74 | N=154 |
No urgency, n (%) | 35 (43.8) | 25 (33.8) | 60 (39.0) |
Urgency, n (%) | 45 (56.3) | 49 (66.2) | 94 (61.0) |
12 months | N=77 | N=56 | N=133 |
No urgency, n (%) | 36 (46.8) | 20 (35.7) | 56 (42.1) |
Urgency, n (%) | 41 (53.2) | 36 (64.3) | 77 (57.9) |
N=total number of patients; n = Number of patients with non-missing observations; TNFi: Tumor necrosis factor inhibitors (adalimumab, golimumab, and infliximab); Non-TNFi: Non-Tumor necrosis factor inhibitors (ustekinumab, vedolizumab, and tofacitinib). No data imputation for missing data was performed in this study. | |||
Disclosures:
James Lewis, MD, MSCE1, Wai Man Maria Chan, PharmD, MPH2, Richard E. Moses, DO, JD2, Xian Zhou, MSc3, Yitong Wang, MS4, Theresa Hunter Gibble, PhD, MPH2. P0745 - Real-World Effectiveness of Advanced Therapies on Bowel Urgency in Patients With Ulcerative Colitis in the United States: Results from SPARC IBD, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
