Sunday Poster Session
Category: IBD
P0814 - Secukinumab Induced Inflammatory Bowel Disease Masquerading as an Infection
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Jessica Basso, MD
Brooke Army Medical Center
Fort Sam Houston, TX
Presenting Author(s)
Jessica Basso, MD1, Cassandra Craig, MD2, Kendra Stilwell, DO1, Jason Hwang, MD1
1Brooke Army Medical Center, Fort Sam Houston, TX; 2San Antonio Military Medical Center, Fort Sam Houston, TX
Introduction: Secukinumab is a human monoclonal antibody binding to interleukin-17A, highly effective in the treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis. Well recognized potential adverse gastrointestinal (GI) events include new-onset inflammatory bowel disease (IBD) or exacerbations, almost exclusively reported in rheumatology, dermatology or drug research journals. Underreported in GI literature is phenotypic presentation, specifically endoscopic features. Our patient's clinical course was highly suggestive of an infectious etiology, but pathology was consistent with Secukinumab induced IBD, fully responsive to mainstay therapy.
Case Description/Methods: We present a 19-year-old male with a history of psoriasis on Secukinumab with 10 days of fevers, oral ulcers, abdominal pain, hematochezia, and fecal urgency. He denied tobacco use, high risk sexual practices or pertinent family history. CT scan was notable for diffuse colonic inflammatory changes. Exam revealed oral ulcers, white plaques on the dorsal tongue surface and supraclavicular lymphadenopathy. On admission he became oral intolerant with labs notable for elevated inflammatory markers, mild leukocytosis with negative infectious work up. Repeat CT was unremarkable. On endoscopic evaluation, esophagitis dissecans was noted along with two discrete 3-5mm esophageal ulcers, patchy, erythematous gastropathy and mild duodenopathy in the bulb. Flexible sigmoidoscopy showed a perianal ulcer and patchy, friable, erythematous, and edematous mucosa throughout the left colon with superficial ulcerations and areas of normal intervening mucosa. Biopsies revealed non-caseating granulomas in the esophagus and colonic apoptosis, most consistent with early Secukinumab induced IBD. 24 hours after initiation of steroids his symptoms were markedly improved. Secukinumab was discontinued and patient was induced with Ustekinumab. At follow up several weeks later all clinical symptoms had resolved.
Discussion: This case highlights the atypical clinical presentation and endoscopic features that can be seen in Secukinumab induced IBD. It is not entirely understood why IBD can present after IL-17 inhibition in some patients. We encourage providers to consider personal and family history risk factors prior to initiating this drug with low threshold to refer for endoscopy if symptoms develop. The utility in inflammatory markers and endoscopy prior to starting Secukinumab in average risk patients is yet to be determined but worth further consideration.

Disclosures:
Jessica Basso, MD1, Cassandra Craig, MD2, Kendra Stilwell, DO1, Jason Hwang, MD1. P0814 - Secukinumab Induced Inflammatory Bowel Disease Masquerading as an Infection, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Brooke Army Medical Center, Fort Sam Houston, TX; 2San Antonio Military Medical Center, Fort Sam Houston, TX
Introduction: Secukinumab is a human monoclonal antibody binding to interleukin-17A, highly effective in the treatment of psoriasis, psoriatic arthritis, and ankylosing spondylitis. Well recognized potential adverse gastrointestinal (GI) events include new-onset inflammatory bowel disease (IBD) or exacerbations, almost exclusively reported in rheumatology, dermatology or drug research journals. Underreported in GI literature is phenotypic presentation, specifically endoscopic features. Our patient's clinical course was highly suggestive of an infectious etiology, but pathology was consistent with Secukinumab induced IBD, fully responsive to mainstay therapy.
Case Description/Methods: We present a 19-year-old male with a history of psoriasis on Secukinumab with 10 days of fevers, oral ulcers, abdominal pain, hematochezia, and fecal urgency. He denied tobacco use, high risk sexual practices or pertinent family history. CT scan was notable for diffuse colonic inflammatory changes. Exam revealed oral ulcers, white plaques on the dorsal tongue surface and supraclavicular lymphadenopathy. On admission he became oral intolerant with labs notable for elevated inflammatory markers, mild leukocytosis with negative infectious work up. Repeat CT was unremarkable. On endoscopic evaluation, esophagitis dissecans was noted along with two discrete 3-5mm esophageal ulcers, patchy, erythematous gastropathy and mild duodenopathy in the bulb. Flexible sigmoidoscopy showed a perianal ulcer and patchy, friable, erythematous, and edematous mucosa throughout the left colon with superficial ulcerations and areas of normal intervening mucosa. Biopsies revealed non-caseating granulomas in the esophagus and colonic apoptosis, most consistent with early Secukinumab induced IBD. 24 hours after initiation of steroids his symptoms were markedly improved. Secukinumab was discontinued and patient was induced with Ustekinumab. At follow up several weeks later all clinical symptoms had resolved.
Discussion: This case highlights the atypical clinical presentation and endoscopic features that can be seen in Secukinumab induced IBD. It is not entirely understood why IBD can present after IL-17 inhibition in some patients. We encourage providers to consider personal and family history risk factors prior to initiating this drug with low threshold to refer for endoscopy if symptoms develop. The utility in inflammatory markers and endoscopy prior to starting Secukinumab in average risk patients is yet to be determined but worth further consideration.

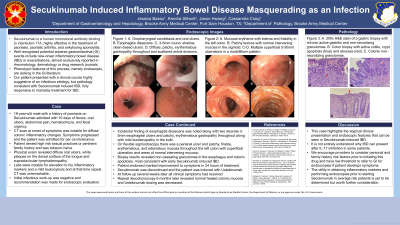
Figure: Figure 1: EGD images with reported histologic findings A-D. A. Oropharyngeal candidiasis and oral ulcers. B. Esophagitis dissecans. C. Acute on chronic inflammation in the distal esophagus. D. Non-caseating granulomas throughout the stomach. CSP images with reported histologic findings. E-H. Colonic mucosa with crypt apoptosis, moderately active colitis, and few non-necrotizing granulomas.
Disclosures:
Jessica Basso indicated no relevant financial relationships.
Cassandra Craig indicated no relevant financial relationships.
Kendra Stilwell indicated no relevant financial relationships.
Jason Hwang indicated no relevant financial relationships.
Jessica Basso, MD1, Cassandra Craig, MD2, Kendra Stilwell, DO1, Jason Hwang, MD1. P0814 - Secukinumab Induced Inflammatory Bowel Disease Masquerading as an Infection, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
