Sunday Poster Session
Category: IBD
P0818 - Unveiling of Crohn's Colitis in a Patient With Rheumatoid Arthritis, Psoriatic Arthritis, and Psoriasis Previously Treated With Etanercept and IL-17 Inhibitors
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
- SA
Sudharshan Achalu, BA
Stanford University School of Medicine
Redwood City, California
Presenting Author(s)
Sudharshan Achalu, BA1, Sandy Huynh, BS2, Rani Berry, MD1, John Gubatan, MD3, Alice G. Cheng, MD4
1Stanford University School of Medicine, Redwood City, CA; 2University of Pennsylvania, Philadelphia, PA; 3Stanford School of Medicine, Stanford, CA; 4Stanford School of Medicine, Redwood City, CA
Introduction: Tumor necrosis factor-alpha (TNF-α) and interleukin-17 (IL-17) inhibitors are among the most potent treatments for psoriasis and spondyloarthropathies (SpA). Though generally safe, biologics may contribute to de novo inflammatory bowel disease (IBD) or exacerbations of pre-existing disease. Such paradoxical adverse events (PAE) have been seen in patients on anti-TNF-α inhibitors (etanercept) and IL-17 inhibitors (secukinumab and ixekizumab). We report a patient with psoriasis, rheumatoid arthritis (RA), and SpA who presented with PAE-induced Crohn’s colitis.
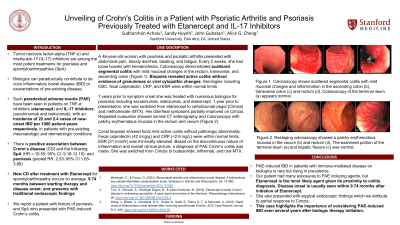
Case Description/Methods: A 46-year-old woman with psoriasis, psoriatic arthritis, and RA presented with abdominal pain, bloody diarrhea, bloating, and fatigue. Every 2 weeks, she had loose bowels with hematochezia. Colonoscopy demonstrated scattered segmental colitis with mild mucosal changes in the rectum, transverse, and ascending colon (Figure 1). Biopsies revealed active colitis without evidence of granulomas or viral cytopathic changes. Serologies including CBC, fecal calprotectin, CRP, and ESR were within normal limits.
7 years prior to symptom onset she was treated with numerous biologics for psoriasis and RA including secukinumab, ixekizumab, and etanercept. 1 year prior to presentation, she was switched from etanercept to certolizumab pegol (Cimzia) and methotrexate (MTX). Her diarrheal symptoms partially improved on Cimzia. Repeated evaluation showed normal CT enterography and Colonoscopy with patchy erythematous mucosa in the rectum and cecum (Figure 2). Cecal biopsies showed focal mild active colitis without pathologic abnormality. Fecal calprotectin (42 mcg/g) and CRP (< 2.9 mg/L) were within normal limits. ESR (21 mm/hr) was minimally elevated. Based on the discontinuous nature of inflammation and overall clinical picture, a diagnosis of PAE Crohn’s colitis was made. She was switched from Cimzia to budesonide, infliximab, and oral MTX.
Discussion: PAE-induced IBD in patients with immune-mediated disease on biologics is rare, but rising in prevalence. Our patient had many exposures to PAE inducing agents, but Etanercept is the most likely agent given its proximity to Colitis diagnosis. Disease onset is usually seen within 5.84 months after initiation of Etanercept. She also presented with atypical endoscopic findings which we attribute to partial response to Cimzia. This case highlights the importance of considering PAE-induced IBD even several years after biologic therapy initiation.

Disclosures:
Sudharshan Achalu, BA1, Sandy Huynh, BS2, Rani Berry, MD1, John Gubatan, MD3, Alice G. Cheng, MD4. P0818 - Unveiling of Crohn's Colitis in a Patient With Rheumatoid Arthritis, Psoriatic Arthritis, and Psoriasis Previously Treated With Etanercept and IL-17 Inhibitors, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Stanford University School of Medicine, Redwood City, CA; 2University of Pennsylvania, Philadelphia, PA; 3Stanford School of Medicine, Stanford, CA; 4Stanford School of Medicine, Redwood City, CA
Introduction: Tumor necrosis factor-alpha (TNF-α) and interleukin-17 (IL-17) inhibitors are among the most potent treatments for psoriasis and spondyloarthropathies (SpA). Though generally safe, biologics may contribute to de novo inflammatory bowel disease (IBD) or exacerbations of pre-existing disease. Such paradoxical adverse events (PAE) have been seen in patients on anti-TNF-α inhibitors (etanercept) and IL-17 inhibitors (secukinumab and ixekizumab). We report a patient with psoriasis, rheumatoid arthritis (RA), and SpA who presented with PAE-induced Crohn’s colitis.
Case Description/Methods: A 46-year-old woman with psoriasis, psoriatic arthritis, and RA presented with abdominal pain, bloody diarrhea, bloating, and fatigue. Every 2 weeks, she had loose bowels with hematochezia. Colonoscopy demonstrated scattered segmental colitis with mild mucosal changes in the rectum, transverse, and ascending colon (Figure 1). Biopsies revealed active colitis without evidence of granulomas or viral cytopathic changes. Serologies including CBC, fecal calprotectin, CRP, and ESR were within normal limits.
7 years prior to symptom onset she was treated with numerous biologics for psoriasis and RA including secukinumab, ixekizumab, and etanercept. 1 year prior to presentation, she was switched from etanercept to certolizumab pegol (Cimzia) and methotrexate (MTX). Her diarrheal symptoms partially improved on Cimzia. Repeated evaluation showed normal CT enterography and Colonoscopy with patchy erythematous mucosa in the rectum and cecum (Figure 2). Cecal biopsies showed focal mild active colitis without pathologic abnormality. Fecal calprotectin (42 mcg/g) and CRP (< 2.9 mg/L) were within normal limits. ESR (21 mm/hr) was minimally elevated. Based on the discontinuous nature of inflammation and overall clinical picture, a diagnosis of PAE Crohn’s colitis was made. She was switched from Cimzia to budesonide, infliximab, and oral MTX.
Discussion: PAE-induced IBD in patients with immune-mediated disease on biologics is rare, but rising in prevalence. Our patient had many exposures to PAE inducing agents, but Etanercept is the most likely agent given its proximity to Colitis diagnosis. Disease onset is usually seen within 5.84 months after initiation of Etanercept. She also presented with atypical endoscopic findings which we attribute to partial response to Cimzia. This case highlights the importance of considering PAE-induced IBD even several years after biologic therapy initiation.

Figure: Figure 1. Colonoscopy shows scattered segmental colitis with mild mucosal changes and inflammation in the ascending colon (b), transverse colon (c) and rectum (d). Colonoscopy of the terminal ileum (a) appears normal.
Figure 2. Restage Colonoscopy showed a patchy erythematous mucosa in the cecum (b) and rectum (d). The examined portion of the terminal ileum (a) and hepatic flexure (c) was normal.
Figure 2. Restage Colonoscopy showed a patchy erythematous mucosa in the cecum (b) and rectum (d). The examined portion of the terminal ileum (a) and hepatic flexure (c) was normal.
Disclosures:
Sudharshan Achalu indicated no relevant financial relationships.
Sandy Huynh indicated no relevant financial relationships.
Rani Berry: metame – Advisor or Review Panel Member.
John Gubatan indicated no relevant financial relationships.
Alice Cheng indicated no relevant financial relationships.
Sudharshan Achalu, BA1, Sandy Huynh, BS2, Rani Berry, MD1, John Gubatan, MD3, Alice G. Cheng, MD4. P0818 - Unveiling of Crohn's Colitis in a Patient With Rheumatoid Arthritis, Psoriatic Arthritis, and Psoriasis Previously Treated With Etanercept and IL-17 Inhibitors, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
