Sunday Poster Session
Category: Liver
P1135 - Leflunomide-Induced Liver Injury
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Tzu-Yu Liu, MD
University of Tennessee Health Science Center/Methodist University
Louisville, KY
Presenting Author(s)
Tzu-Yu Liu, MD1, Dawit Jowhar, MD, PhD2, Hemnishil Marella, DO2, Nair Satheesh, MD3, Jiten Kothadia, MD4
1University of Tennessee Health Science Center/Methodist University, Louisville, KY; 2University of Tennessee Health Science Center, Memphis, TN; 3University of Tennessee Health Science Center/Methodist University Hospital, Memphis, TN; 4University of Tennessee Health Science Center/Methodist University, Memphis, TN
Introduction: Leflunomide (LF) is a disease-modifying antirheumatic drug (DMARD) used widely in the United States with approximately one million prescriptions per year from 2016 to 2019. LF is used to treat autoimmune arthritis, predominantly rheumatoid and psoriatic arthritis. LF has been reported to cause rare instances of clinically apparent acute liver injury. We report a case of LF-induced acute liver injury.
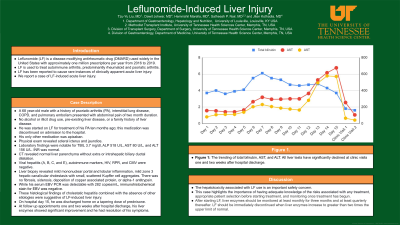
Case Description/Methods: A 68 year-old male with a history of psoriatic arthritis (PA), interstitial lung disease, COPD, and pulmonary embolism presented with abdominal pain of two month duration. He denied alcohol or illicit drug use, pre-existing liver disease, or a family history of liver disease. He was started on LF for treatment of his PA ten months ago; this medication was discontinued on admission to the hospital. His only other medication was apixaban. A physical exam revealed scleral icterus and jaundice. Laboratory findings were notable for TBIL 3.7 mg/dl, ALP 518 U/L, AST 80 U/L, and ALT 156 U/L (Figure 1). INR was normal. Imaging with CT revealed normal liver parenchyma without extra or intrahepatic biliary ductal dilatation. Viral hepatitis (A, B, C, and E), autoimmune markers, HIV, RPR, and CMV were negative. He underwent liver biopsy which revealed mild mononuclear portal and lobular inflammation, mild zone 3 hepatic canalicular cholestasis with small, scattered Kupffer cell aggregates. There was no fibrosis, siderosis, deposition of copper associated protein, or alpha-1 antitrypsin. While his serum EBV PCR was detectable with 282 copies/mL, immunohistochemical stain for EBV was negative. These histological findings of cholestatic hepatitis combined with the absence of other etiologies were suggestive of LF-induced liver injury. He was discharged home on a tapering dose of prednisone. The patient followed up in the outpatient clinic. At follow up appointments one and two weeks after hospital discharge, his liver enzymes showed significant improvement and he had resolution of his symptoms.
Discussion: The hepatotoxicity associated with LF use is an important safety concern that physicians should be aware of. This case highlights the importance of appropriate patient selection before initiating treatment and monitoring once treatment has begun. After starting LF, liver enzymes should be monitored at least monthly for three months and at least quarterly thereafter. LF should be discontinued when liver enzymes increase to greater than two times the upper limit of normal.

Disclosures:
Tzu-Yu Liu, MD1, Dawit Jowhar, MD, PhD2, Hemnishil Marella, DO2, Nair Satheesh, MD3, Jiten Kothadia, MD4. P1135 - Leflunomide-Induced Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Tennessee Health Science Center/Methodist University, Louisville, KY; 2University of Tennessee Health Science Center, Memphis, TN; 3University of Tennessee Health Science Center/Methodist University Hospital, Memphis, TN; 4University of Tennessee Health Science Center/Methodist University, Memphis, TN
Introduction: Leflunomide (LF) is a disease-modifying antirheumatic drug (DMARD) used widely in the United States with approximately one million prescriptions per year from 2016 to 2019. LF is used to treat autoimmune arthritis, predominantly rheumatoid and psoriatic arthritis. LF has been reported to cause rare instances of clinically apparent acute liver injury. We report a case of LF-induced acute liver injury.
Case Description/Methods: A 68 year-old male with a history of psoriatic arthritis (PA), interstitial lung disease, COPD, and pulmonary embolism presented with abdominal pain of two month duration. He denied alcohol or illicit drug use, pre-existing liver disease, or a family history of liver disease. He was started on LF for treatment of his PA ten months ago; this medication was discontinued on admission to the hospital. His only other medication was apixaban. A physical exam revealed scleral icterus and jaundice. Laboratory findings were notable for TBIL 3.7 mg/dl, ALP 518 U/L, AST 80 U/L, and ALT 156 U/L (Figure 1). INR was normal. Imaging with CT revealed normal liver parenchyma without extra or intrahepatic biliary ductal dilatation. Viral hepatitis (A, B, C, and E), autoimmune markers, HIV, RPR, and CMV were negative. He underwent liver biopsy which revealed mild mononuclear portal and lobular inflammation, mild zone 3 hepatic canalicular cholestasis with small, scattered Kupffer cell aggregates. There was no fibrosis, siderosis, deposition of copper associated protein, or alpha-1 antitrypsin. While his serum EBV PCR was detectable with 282 copies/mL, immunohistochemical stain for EBV was negative. These histological findings of cholestatic hepatitis combined with the absence of other etiologies were suggestive of LF-induced liver injury. He was discharged home on a tapering dose of prednisone. The patient followed up in the outpatient clinic. At follow up appointments one and two weeks after hospital discharge, his liver enzymes showed significant improvement and he had resolution of his symptoms.
Discussion: The hepatotoxicity associated with LF use is an important safety concern that physicians should be aware of. This case highlights the importance of appropriate patient selection before initiating treatment and monitoring once treatment has begun. After starting LF, liver enzymes should be monitored at least monthly for three months and at least quarterly thereafter. LF should be discontinued when liver enzymes increase to greater than two times the upper limit of normal.

Figure: Figure 1. The trending of total bilirubin, AST, and ALT. All liver tests have significantly declined at clinic visits one and two weeks after hospital discharge.
Disclosures:
Tzu-Yu Liu indicated no relevant financial relationships.
Dawit Jowhar indicated no relevant financial relationships.
Hemnishil Marella indicated no relevant financial relationships.
Nair Satheesh indicated no relevant financial relationships.
Jiten Kothadia indicated no relevant financial relationships.
Tzu-Yu Liu, MD1, Dawit Jowhar, MD, PhD2, Hemnishil Marella, DO2, Nair Satheesh, MD3, Jiten Kothadia, MD4. P1135 - Leflunomide-Induced Liver Injury, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
