Sunday Poster Session
Category: Liver
P1143 - Drug-Induced Autoimmune Hepatitis: A Rare Complication of Infliximab Therapy in the Setting of Inflammatory Bowel Disease
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Parvir Aujla, MD
University of Texas Health Science Center
Houston, TX
Presenting Author(s)
Parvir Aujla, MD1, Yllen Hernandez Blanco, MD2, Erin Rubin, MD3, Victor Machicao, MD2, Tanima Jana, MD2
1University of Texas Health Science Center, Houston, TX; 2University of Texas, Houston, TX; 3McGovern Medical School, Houston, TX
Introduction: Drug-induced autoimmune hepatitis (DI-AIH) is a rare side effect of infliximab therapy, and only a paucity of case reports exist. We describe a case of a patient with ulcerative pancolitis presenting with severe acute hepatitis who was diagnosed with DI-AIH due to infliximab, and had a favorable response to corticosteroids and azathioprine.
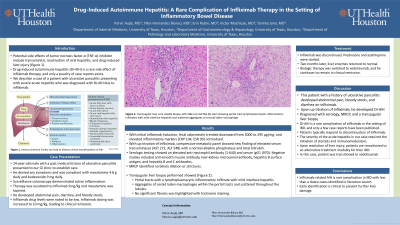
Case Description/Methods: Our patient is a 24-year-old man with a history of ulcerative pancolitis with symptoms well-controlled on mesalamine. Routine surveillance colonoscopy demonstrated active inflammation throughout the colon. Infliximab 5 mg/kg was initiated and mesalamine was discontinued. Fecal calprotectin trended downward from 5000 to 295 μg/mg, and elevated inflammatory markers (CRP 104, ESR 99) normalized. Unfortunately, his symptoms recurred. Infliximab drug levels were noted to be low. Infliximab dosing was increased to 10 mg/kg, leading to clinical remission. Routine labs showed a new finding of elevated serum transaminases (AST 272, ALT 548) with a normal alkaline phosphatase and total bilirubin. Additional serologic testing showed an elevated anti-neutrophil antibody (1:640) and serum IgG1 (970). Negative studies included anti-smooth muscle antibody, liver-kidney microsomal antibody, hepatitis B surface antigen, and hepatitis B and C antibodies. No biliary dilation or strictures were identified on MRCP. Transjugular liver biopsy showed portal tracts with a lymphoplasmacytic inflammatory infiltrate with mild interface hepatitis. Both within the portal tracts and scattered throughout the lobules were aggregates of ceroid laden macrophages (Figure 1). No significant fibrosis was highlighted with trichrome staining. Infliximab was discontinued, and prednisone and azathioprine started. Two months later, liver enzymes returned to normal. Biologic therapy was switched to vedolizumab, and he remained in clinical remission.
Discussion: DI-AIH is a rare complication of infliximab in the setting of IBD, and only a few case reports have been published. Patients typically respond to discontinuation of infliximab. The severity of the acute hepatitis in our case required the initiation of steroids and immunomodulators. Upon resolution of liver injury, patients are transitioned to an alternative treatment modality for their IBD. In summary, the early identification of infliximab-related AIH is critical to prevent further liver damage, and immunomodulator therapy is beneficial in most severe cases.

Disclosures:
Parvir Aujla, MD1, Yllen Hernandez Blanco, MD2, Erin Rubin, MD3, Victor Machicao, MD2, Tanima Jana, MD2. P1143 - Drug-Induced Autoimmune Hepatitis: A Rare Complication of Infliximab Therapy in the Setting of Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Texas Health Science Center, Houston, TX; 2University of Texas, Houston, TX; 3McGovern Medical School, Houston, TX
Introduction: Drug-induced autoimmune hepatitis (DI-AIH) is a rare side effect of infliximab therapy, and only a paucity of case reports exist. We describe a case of a patient with ulcerative pancolitis presenting with severe acute hepatitis who was diagnosed with DI-AIH due to infliximab, and had a favorable response to corticosteroids and azathioprine.
Case Description/Methods: Our patient is a 24-year-old man with a history of ulcerative pancolitis with symptoms well-controlled on mesalamine. Routine surveillance colonoscopy demonstrated active inflammation throughout the colon. Infliximab 5 mg/kg was initiated and mesalamine was discontinued. Fecal calprotectin trended downward from 5000 to 295 μg/mg, and elevated inflammatory markers (CRP 104, ESR 99) normalized. Unfortunately, his symptoms recurred. Infliximab drug levels were noted to be low. Infliximab dosing was increased to 10 mg/kg, leading to clinical remission. Routine labs showed a new finding of elevated serum transaminases (AST 272, ALT 548) with a normal alkaline phosphatase and total bilirubin. Additional serologic testing showed an elevated anti-neutrophil antibody (1:640) and serum IgG1 (970). Negative studies included anti-smooth muscle antibody, liver-kidney microsomal antibody, hepatitis B surface antigen, and hepatitis B and C antibodies. No biliary dilation or strictures were identified on MRCP. Transjugular liver biopsy showed portal tracts with a lymphoplasmacytic inflammatory infiltrate with mild interface hepatitis. Both within the portal tracts and scattered throughout the lobules were aggregates of ceroid laden macrophages (Figure 1). No significant fibrosis was highlighted with trichrome staining. Infliximab was discontinued, and prednisone and azathioprine started. Two months later, liver enzymes returned to normal. Biologic therapy was switched to vedolizumab, and he remained in clinical remission.
Discussion: DI-AIH is a rare complication of infliximab in the setting of IBD, and only a few case reports have been published. Patients typically respond to discontinuation of infliximab. The severity of the acute hepatitis in our case required the initiation of steroids and immunomodulators. Upon resolution of liver injury, patients are transitioned to an alternative treatment modality for their IBD. In summary, the early identification of infliximab-related AIH is critical to prevent further liver damage, and immunomodulator therapy is beneficial in most severe cases.

Figure: Figure 1: Transjugular liver core needle biopsy with H&E (a) and PAS (b) stain showing portal tract lymphoplasmacytic inflammatory infiltrates with mild interface hepatitis and scattered aggregates of cercoid laden macrophage.
Disclosures:
Parvir Aujla indicated no relevant financial relationships.
Yllen Hernandez Blanco indicated no relevant financial relationships.
Erin Rubin indicated no relevant financial relationships.
Victor Machicao indicated no relevant financial relationships.
Tanima Jana indicated no relevant financial relationships.
Parvir Aujla, MD1, Yllen Hernandez Blanco, MD2, Erin Rubin, MD3, Victor Machicao, MD2, Tanima Jana, MD2. P1143 - Drug-Induced Autoimmune Hepatitis: A Rare Complication of Infliximab Therapy in the Setting of Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
