Sunday Poster Session
Category: Practice Management
P1237 - Gastroenterology Guidelines Transparency: A Call for Improvement in the United States and Europe
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Laith Numan, MD, MS
Saint Louis University
St. Louis, MO
Presenting Author(s)
Laith Numan, MD, MS1, Thomas J.. Mathews, MD2, Jamie Varghese, BS3, Motib Alabdulwahhab, MD4, Chiara Maruggi, MD5, Mahak Chauhan, MD6, Apoorva Chandar, MBBS, MPH7, Yngve Falck-Ytter, MD8, Perica Davitkov, MD7, Shahnaz Sultan, MD9, Raj Shah, MD10
1Saint Louis University, St. Louis, MO; 2University of Kansas Medical Center, Kansas City, MO; 3St. George's University, St. Louis, MO; 4University Hospitals-Cleveland Medical Center, Cleveland, OH; 5Cleveland Clinic, Cleveland, OH; 6University of Arizona-Tucson College of Medicine, Tucson, AZ; 7Case Western Reserve University, Cleveland, OH; 8Case Western Reserve/Louis Stokes Cleveland VAMC, Cleveland, OH; 9University of Minnesota, Minneapolis, MN; 10Brigham and Women's Hospital, Boston, MA
Introduction: Transparency is a key factor to minimize potential biases such as expert or industrial influences in Clinical Practice Guidelines (CPGs). In this study we aim to evaluate the prevalence of COI reporting in gastroenterology (GI) guidelines from the United States and Europe.
Methods: Nine GI societies websites in the US and Europe were reviewed for all published guidelines that used the GRADE methodology from 2013 to October 1, 2022. Guidelines were reviewed by one author to assess COI self-reporting, and COI details for authors. The conflicts were categorized as “research related payments”, “direct Payments” or “mixed: direct payments and research payments”. “Direct payments” conflicts such as speaker's bureau, stocks, consulting, advisory board, honoraria, non-research funding, and industry-sponsored continuing medical education activities. All included guidelines were assessed for publishing the evidence profile as another measure for transparency.
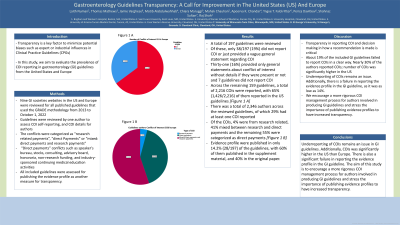
Results: A total of 197 guidelines were reviewed. Of these, only 38/197 (19%) did not report COI or just provided a vague general statement regarding COI; Thirty-one (16%) provided only general statements about conflict of interest without details if they were present or not and 7 guidelines did not report COI. Across the remaining 159 guidelines, a total of 2,216 COIs were reported, with 65% (1,426/2,216) of them reported in the US guidelines [Figure 1]. There was a total of 2,346 authors across the reviewed guidelines, of which 29% had at least one COI reported. Of the COIs, 4% were from research related, 41% mixed between research and direct payments and the remaining 55% were categorized as direct payments [Figure 2]. Evidence profile were published in only 14.2% (28/197) of the guidelines, with 60% of them published in the supplement material, and 40% in the original paper.
Discussion: Transparency in reporting COI and decision making in how a recommendation is made is critical. About 19% of the included GI guidelines failed to report COIs in a clear way. Nearly 30% of the authors reported COIs; number of COIs was significantly higher in the US. Underreporting of COIs remains an issue. Additionally, there is a failure in reporting the evidence profile in the GI guideline, as it was as low as 14%. Therefore, we encourage a more rigorous COI management process for authors involved in producing GI guidelines and stress the importance of publishing evidence profiles to have increased transparency.

Disclosures:
Laith Numan, MD, MS1, Thomas J.. Mathews, MD2, Jamie Varghese, BS3, Motib Alabdulwahhab, MD4, Chiara Maruggi, MD5, Mahak Chauhan, MD6, Apoorva Chandar, MBBS, MPH7, Yngve Falck-Ytter, MD8, Perica Davitkov, MD7, Shahnaz Sultan, MD9, Raj Shah, MD10. P1237 - Gastroenterology Guidelines Transparency: A Call for Improvement in the United States and Europe, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Saint Louis University, St. Louis, MO; 2University of Kansas Medical Center, Kansas City, MO; 3St. George's University, St. Louis, MO; 4University Hospitals-Cleveland Medical Center, Cleveland, OH; 5Cleveland Clinic, Cleveland, OH; 6University of Arizona-Tucson College of Medicine, Tucson, AZ; 7Case Western Reserve University, Cleveland, OH; 8Case Western Reserve/Louis Stokes Cleveland VAMC, Cleveland, OH; 9University of Minnesota, Minneapolis, MN; 10Brigham and Women's Hospital, Boston, MA
Introduction: Transparency is a key factor to minimize potential biases such as expert or industrial influences in Clinical Practice Guidelines (CPGs). In this study we aim to evaluate the prevalence of COI reporting in gastroenterology (GI) guidelines from the United States and Europe.
Methods: Nine GI societies websites in the US and Europe were reviewed for all published guidelines that used the GRADE methodology from 2013 to October 1, 2022. Guidelines were reviewed by one author to assess COI self-reporting, and COI details for authors. The conflicts were categorized as “research related payments”, “direct Payments” or “mixed: direct payments and research payments”. “Direct payments” conflicts such as speaker's bureau, stocks, consulting, advisory board, honoraria, non-research funding, and industry-sponsored continuing medical education activities. All included guidelines were assessed for publishing the evidence profile as another measure for transparency.
Results: A total of 197 guidelines were reviewed. Of these, only 38/197 (19%) did not report COI or just provided a vague general statement regarding COI; Thirty-one (16%) provided only general statements about conflict of interest without details if they were present or not and 7 guidelines did not report COI. Across the remaining 159 guidelines, a total of 2,216 COIs were reported, with 65% (1,426/2,216) of them reported in the US guidelines [Figure 1]. There was a total of 2,346 authors across the reviewed guidelines, of which 29% had at least one COI reported. Of the COIs, 4% were from research related, 41% mixed between research and direct payments and the remaining 55% were categorized as direct payments [Figure 2]. Evidence profile were published in only 14.2% (28/197) of the guidelines, with 60% of them published in the supplement material, and 40% in the original paper.
Discussion: Transparency in reporting COI and decision making in how a recommendation is made is critical. About 19% of the included GI guidelines failed to report COIs in a clear way. Nearly 30% of the authors reported COIs; number of COIs was significantly higher in the US. Underreporting of COIs remains an issue. Additionally, there is a failure in reporting the evidence profile in the GI guideline, as it was as low as 14%. Therefore, we encourage a more rigorous COI management process for authors involved in producing GI guidelines and stress the importance of publishing evidence profiles to have increased transparency.

Figure: Figure 1. A: Guidelines Conflict of Interest In The US Guidelines Versus Europe. B: Guidelines authors' conflict of interest by type.
Disclosures:
Laith Numan indicated no relevant financial relationships.
Thomas Mathews indicated no relevant financial relationships.
Jamie Varghese indicated no relevant financial relationships.
Motib Alabdulwahhab indicated no relevant financial relationships.
Chiara Maruggi indicated no relevant financial relationships.
Mahak Chauhan indicated no relevant financial relationships.
Apoorva Chandar indicated no relevant financial relationships.
Yngve Falck-Ytter indicated no relevant financial relationships.
Perica Davitkov indicated no relevant financial relationships.
Shahnaz Sultan indicated no relevant financial relationships.
Raj Shah indicated no relevant financial relationships.
Laith Numan, MD, MS1, Thomas J.. Mathews, MD2, Jamie Varghese, BS3, Motib Alabdulwahhab, MD4, Chiara Maruggi, MD5, Mahak Chauhan, MD6, Apoorva Chandar, MBBS, MPH7, Yngve Falck-Ytter, MD8, Perica Davitkov, MD7, Shahnaz Sultan, MD9, Raj Shah, MD10. P1237 - Gastroenterology Guidelines Transparency: A Call for Improvement in the United States and Europe, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
