Sunday Poster Session
Category: Small Intestine
P1265 - Irbesartan-Induced Enteropathy: A Case Report Demonstrating Clinical and Histological Improvement With Medication Cessation
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Joann P. Wongvravit, DO
New York-Presbyterian Queens Hospital
Flushing, NY
Presenting Author(s)
Joann P. Wongvravit, DO1, Jason Cohen, MD2, Jaslyn Maurer, DO3, Jiankun Tong, MD1, Jean Luo, MD1, Ellen Gutkin, DO3
1New York-Presbyterian Queens Hospital, Flushing, NY; 2New York-Presbyterian Queens, Queens, NY; 3New York-Presbyterian/Queens, Flushing, NY
Introduction: There has been increasing evidence of association between angiotensin II receptor blocker therapy and enteropathy. A majority of cases have been attributed to olmesartan. The incidence of irbesartan-associated enteropathy is currently unknown. Our case demonstrates irbesartan-associated enteropathy that was discovered on esophagogastroduodenoscopy (EGD) with resolution after 3-month cessation of irbesartan.
Case Description/Methods: A 57-year-old male with hypertension and asthma presented for dyspepsia in 2016. At that time, his hypertension was being managed with irbesartan and amlodipine. Initial EGD in 2016 revealed gastric erosions and duodenal erosions/linear duodenal ulcerations. Six years later, his blood pressure medications remained the same. He underwent a second EGD in 2022 for heartburn which revealed a few erosions at the gastroesophageal junction, gastritis and a few superficial duodenal ulcers. Pathology revealed duodenitis with focal mucosal erosions. Given these findings, irbesartan was discontinued. Three months later, he underwent repeat EGD which demonstrated normal appearing duodenum. Pathology also confirmed normal duodenal mucosa. Since the discontinuation of irbesartan, his symptoms improved and did not respond to a gluten-free diet, however celiac serologies are pending.
Discussion: There have only been five cases reporting irbesartan-associated enteropathy. Of these cases, three were female and two were male with ages between 54 to 80 years old, being treated with irbesartan for 6 to 156 months, and most commonly presented with diarrhea. Their symptoms subsided immediately following discontinuation of irbesartan, one case even demonstrated histological recovery after 3 months. The pathogenesis is not clearly understood, but may be due to an immune-mediated inflammatory response as seen with olmesartan. Our case emphasizes the clinical and histological correlation between irbesartan-induced enteropathy as our patient demonstrated both clinical improvement and histological resolution after cessation of irbesartan for three months. The differential diagnoses for diarrhea should remain broad with a high degree of clinical suspicion for underlying drug-induced gastrointestinal injury, especially in older age groups taking multiple medications. It is prudent that providers remain vigilant about a patient’s medication list as this disease has favorable outcomes if identified and treated appropriately.

Disclosures:
Joann P. Wongvravit, DO1, Jason Cohen, MD2, Jaslyn Maurer, DO3, Jiankun Tong, MD1, Jean Luo, MD1, Ellen Gutkin, DO3. P1265 - Irbesartan-Induced Enteropathy: A Case Report Demonstrating Clinical and Histological Improvement With Medication Cessation, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1New York-Presbyterian Queens Hospital, Flushing, NY; 2New York-Presbyterian Queens, Queens, NY; 3New York-Presbyterian/Queens, Flushing, NY
Introduction: There has been increasing evidence of association between angiotensin II receptor blocker therapy and enteropathy. A majority of cases have been attributed to olmesartan. The incidence of irbesartan-associated enteropathy is currently unknown. Our case demonstrates irbesartan-associated enteropathy that was discovered on esophagogastroduodenoscopy (EGD) with resolution after 3-month cessation of irbesartan.
Case Description/Methods: A 57-year-old male with hypertension and asthma presented for dyspepsia in 2016. At that time, his hypertension was being managed with irbesartan and amlodipine. Initial EGD in 2016 revealed gastric erosions and duodenal erosions/linear duodenal ulcerations. Six years later, his blood pressure medications remained the same. He underwent a second EGD in 2022 for heartburn which revealed a few erosions at the gastroesophageal junction, gastritis and a few superficial duodenal ulcers. Pathology revealed duodenitis with focal mucosal erosions. Given these findings, irbesartan was discontinued. Three months later, he underwent repeat EGD which demonstrated normal appearing duodenum. Pathology also confirmed normal duodenal mucosa. Since the discontinuation of irbesartan, his symptoms improved and did not respond to a gluten-free diet, however celiac serologies are pending.
Discussion: There have only been five cases reporting irbesartan-associated enteropathy. Of these cases, three were female and two were male with ages between 54 to 80 years old, being treated with irbesartan for 6 to 156 months, and most commonly presented with diarrhea. Their symptoms subsided immediately following discontinuation of irbesartan, one case even demonstrated histological recovery after 3 months. The pathogenesis is not clearly understood, but may be due to an immune-mediated inflammatory response as seen with olmesartan. Our case emphasizes the clinical and histological correlation between irbesartan-induced enteropathy as our patient demonstrated both clinical improvement and histological resolution after cessation of irbesartan for three months. The differential diagnoses for diarrhea should remain broad with a high degree of clinical suspicion for underlying drug-induced gastrointestinal injury, especially in older age groups taking multiple medications. It is prudent that providers remain vigilant about a patient’s medication list as this disease has favorable outcomes if identified and treated appropriately.

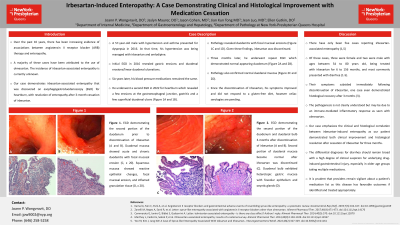
Figure: Figure 1. EGD demonstrating the second portion of the duodenum prior to discontinuation of irbesartan (A and B) and second portion of the duodenum and duodenal bulb 3-months after discontinuation of irbesartan respectively (C and D). Duodenal mucosa showing acute and chronic duodenitis with focal mucosal erosion (E, x 20). Squamous mucosa showing reactive epithelial changes, focal mucosal erosion, and inflamed granulation tissue (F, x 20).
Disclosures:
Joann Wongvravit indicated no relevant financial relationships.
Jason Cohen indicated no relevant financial relationships.
Jaslyn Maurer indicated no relevant financial relationships.
Jiankun Tong indicated no relevant financial relationships.
Jean Luo indicated no relevant financial relationships.
Ellen Gutkin indicated no relevant financial relationships.
Joann P. Wongvravit, DO1, Jason Cohen, MD2, Jaslyn Maurer, DO3, Jiankun Tong, MD1, Jean Luo, MD1, Ellen Gutkin, DO3. P1265 - Irbesartan-Induced Enteropathy: A Case Report Demonstrating Clinical and Histological Improvement With Medication Cessation, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

