Sunday Poster Session
Category: Stomach
P1357 - A Case of Iron Pill-Induced Gastritis
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio
.jpg)
Mudassar Sandozi, DO
MercyHealth
Rockford, IL
Presenting Author(s)
Mudassar Sandozi, DO, Ahmet Sakiri, MD, Thayer Hamoudah, MD, Altaf Dawood, MD, MBBS, Naser Khan, MD
MercyHealth, Rockford, IL
Introduction: Ferrous Sulfate is a commonly used medication to treat iron deficiency anemia. Though generally considered a safe medication with mild side effects, mucosal injury and upper GI bleeds can occur from its administration. We present a case of ferrous sulfate induced iron-pill gastritis in a patient with severe cardiovascular comorbidities.
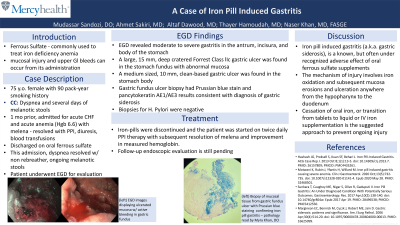
Case Description/Methods: A 75-year-old female with multiple medical comorbidities and a 90 pack-year smoking history presented with dyspnea. One month prior, patient was admitted for a CHF exacerbation in the presence of acute blood loss anemia with melena and a hemoglobin of 6.6. Symptoms resolved with PPI’s, diuresis, and blood transfusions. All home medications were resumed on discharge, with the addition oral iron tablets. Patient was now admitted for respiratory distress that resolved with a non-rebreather. On presentation, she endorsed several days of melanotic stools. Patient underwent EGD that revealed moderate to severe gastritis in the antrum, incisura, and the body of the stomach. A large, 15 mm, deep cratered Forrest Class IIc gastric ulcer was found in the stomach fundus with abnormal mucosa. Another medium sized, 10 mm, clean-based gastric ulcer was found in the stomach body. Biopsies of the ulcers and random biopsies were obtained for H. Pylori testing. Gastric fundus ulcer biopsy had Prussian blue stain and pancytokeratin AE1/AE3 results consistent with diagnosis of gastric siderosis. Biopsies for H. Pylori were negative. Iron-pills were discontinued and the patient was started on twice daily PPI therapy with subsequent resolution of melena and improvement in measured hemoglobin. Follow-up endoscopic evaluation is still pending.
Discussion: Iron pill induced gastritis (a.k.a. gastric siderosis), is a known, but often under recognized adverse effect of oral ferrous sulfate supplements. The mechanism of injury involves iron oxidation and subsequent mucosal erosions and ulceration anywhere from the hypopharynx to the duodenum. Cessation of oral iron, or transition from tablets to liquid or IV iron supplementation is the suggested approach to prevent ongoing injury. The purpose of this abstract is to remind gastroenterologist of the potential risks associated with iron-pill ingestion and to highlight alternative treatments available when complications are encountered.

Disclosures:
Mudassar Sandozi, DO, Ahmet Sakiri, MD, Thayer Hamoudah, MD, Altaf Dawood, MD, MBBS, Naser Khan, MD. P1357 - A Case of Iron Pill-Induced Gastritis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
MercyHealth, Rockford, IL
Introduction: Ferrous Sulfate is a commonly used medication to treat iron deficiency anemia. Though generally considered a safe medication with mild side effects, mucosal injury and upper GI bleeds can occur from its administration. We present a case of ferrous sulfate induced iron-pill gastritis in a patient with severe cardiovascular comorbidities.
Case Description/Methods: A 75-year-old female with multiple medical comorbidities and a 90 pack-year smoking history presented with dyspnea. One month prior, patient was admitted for a CHF exacerbation in the presence of acute blood loss anemia with melena and a hemoglobin of 6.6. Symptoms resolved with PPI’s, diuresis, and blood transfusions. All home medications were resumed on discharge, with the addition oral iron tablets. Patient was now admitted for respiratory distress that resolved with a non-rebreather. On presentation, she endorsed several days of melanotic stools. Patient underwent EGD that revealed moderate to severe gastritis in the antrum, incisura, and the body of the stomach. A large, 15 mm, deep cratered Forrest Class IIc gastric ulcer was found in the stomach fundus with abnormal mucosa. Another medium sized, 10 mm, clean-based gastric ulcer was found in the stomach body. Biopsies of the ulcers and random biopsies were obtained for H. Pylori testing. Gastric fundus ulcer biopsy had Prussian blue stain and pancytokeratin AE1/AE3 results consistent with diagnosis of gastric siderosis. Biopsies for H. Pylori were negative. Iron-pills were discontinued and the patient was started on twice daily PPI therapy with subsequent resolution of melena and improvement in measured hemoglobin. Follow-up endoscopic evaluation is still pending.
Discussion: Iron pill induced gastritis (a.k.a. gastric siderosis), is a known, but often under recognized adverse effect of oral ferrous sulfate supplements. The mechanism of injury involves iron oxidation and subsequent mucosal erosions and ulceration anywhere from the hypopharynx to the duodenum. Cessation of oral iron, or transition from tablets to liquid or IV iron supplementation is the suggested approach to prevent ongoing injury. The purpose of this abstract is to remind gastroenterologist of the potential risks associated with iron-pill ingestion and to highlight alternative treatments available when complications are encountered.

Figure: EGD visualization of iron-pill induced gastric ulcer
Disclosures:
Mudassar Sandozi indicated no relevant financial relationships.
Ahmet Sakiri indicated no relevant financial relationships.
Thayer Hamoudah indicated no relevant financial relationships.
Altaf Dawood indicated no relevant financial relationships.
Naser Khan indicated no relevant financial relationships.
Mudassar Sandozi, DO, Ahmet Sakiri, MD, Thayer Hamoudah, MD, Altaf Dawood, MD, MBBS, Naser Khan, MD. P1357 - A Case of Iron Pill-Induced Gastritis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
