Sunday Poster Session
Category: Stomach
P1416 - Beyond the Esophagus: Dupilumab’s Potential in Treatment of Eosinophilic Gastritis
Sunday, October 22, 2023
3:30 PM - 7:00 PM PT
Location: Exhibit Hall
Has Audio

Braden Thomas, MD
Houston Methodist Hospital
Houston, TX
Presenting Author(s)
Braden Thomas, MD, Neha Mathur, MD
Houston Methodist Hospital, Houston, TX
Introduction: Dupilumab is a human monoclonal antibody targeted against interleukin (IL)-4 and IL-13 signaling. Blocking these receptors leads to a suppression of type 2 inflammation, making it an option for associated inflammatory conditions like Eosinophilic Esophagitis (EOE). EOE is a chronic immune or antigen-mediated process characterized by mucosal inflammation with predominant eosinophils. As the name implies, mucosal inflammation in EOE is confined to the esophagus only (2). However, eosinophilic infiltration can rarely affect the stomach, known as eosinophilic gastritis (EOG), or the stomach and intestines, known as eosinophilic gastroenteritis (EGE) (3). Dupilimab is approved for treatment of EOE but is not approved for treatment of EOG or EGE. The following case describes a patient with both EOE and EOG complicated by chronic gastric ulceration that was successfully treated with dupilumab.
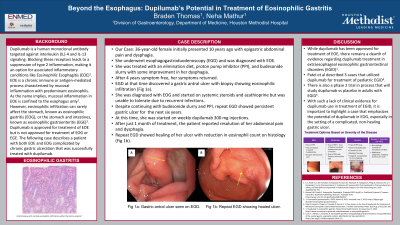
Case Description/Methods: A 34-year-old female presented 10 years ago with epigastric abdominal pain and dysphagia. She underwent esophagogastroduodenoscopy (EGD) and was diagnosed with EOE. She was treated with an elimination diet, proton pump inhibitor (PPI), and budesonide slurry with some improvement in her dysphagia. After 4 years, her symptoms returned. EGD at that time discovered a gastric antral ulcer with biopsy showing eosinophilic infiltration (Fig 1a). She was diagnosed with EOG and started on systemic steroids and azathioprine but was unable to tolerate due to recurrent infections. Despite continuing with budesonide slurry and PPI, repeat EGD showed persistent gastric ulcer for over the next 6 years. At this time, she was started on weekly dupilumab 300 mg subcutaneous injections. After just 1 month of treatment, the patient reported resolution of her abdominal pain and dysphagia. Repeat EGD showed healing of her ulcer with reduction in eosinophil count on histology (Fig 1b).
Discussion: While dupilumab has been approved for treatment of EOE, there remains a dearth of evidence regarding dupilumab treatment in extraesophageal eosinophilic gastrointestinal disorders (EGID) (1). Patel et al described 3 cases that utilized dupilumab for treatment of pediatric EGID (4). There is also a phase 2 trial in process that will study dupilumab vs placebo in adults with EGID (5). With such a lack of clinical evidence for dupilumab use in EGID, it is important to highlight a case that emphasizes the potential of dupilumab in treatment of EOG, especially in the setting of a complicated non-healing gastric ulcer.

Disclosures:
Braden Thomas, MD, Neha Mathur, MD. P1416 - Beyond the Esophagus: Dupilumab’s Potential in Treatment of Eosinophilic Gastritis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Houston Methodist Hospital, Houston, TX
Introduction: Dupilumab is a human monoclonal antibody targeted against interleukin (IL)-4 and IL-13 signaling. Blocking these receptors leads to a suppression of type 2 inflammation, making it an option for associated inflammatory conditions like Eosinophilic Esophagitis (EOE). EOE is a chronic immune or antigen-mediated process characterized by mucosal inflammation with predominant eosinophils. As the name implies, mucosal inflammation in EOE is confined to the esophagus only (2). However, eosinophilic infiltration can rarely affect the stomach, known as eosinophilic gastritis (EOG), or the stomach and intestines, known as eosinophilic gastroenteritis (EGE) (3). Dupilimab is approved for treatment of EOE but is not approved for treatment of EOG or EGE. The following case describes a patient with both EOE and EOG complicated by chronic gastric ulceration that was successfully treated with dupilumab.
Case Description/Methods: A 34-year-old female presented 10 years ago with epigastric abdominal pain and dysphagia. She underwent esophagogastroduodenoscopy (EGD) and was diagnosed with EOE. She was treated with an elimination diet, proton pump inhibitor (PPI), and budesonide slurry with some improvement in her dysphagia. After 4 years, her symptoms returned. EGD at that time discovered a gastric antral ulcer with biopsy showing eosinophilic infiltration (Fig 1a). She was diagnosed with EOG and started on systemic steroids and azathioprine but was unable to tolerate due to recurrent infections. Despite continuing with budesonide slurry and PPI, repeat EGD showed persistent gastric ulcer for over the next 6 years. At this time, she was started on weekly dupilumab 300 mg subcutaneous injections. After just 1 month of treatment, the patient reported resolution of her abdominal pain and dysphagia. Repeat EGD showed healing of her ulcer with reduction in eosinophil count on histology (Fig 1b).
Discussion: While dupilumab has been approved for treatment of EOE, there remains a dearth of evidence regarding dupilumab treatment in extraesophageal eosinophilic gastrointestinal disorders (EGID) (1). Patel et al described 3 cases that utilized dupilumab for treatment of pediatric EGID (4). There is also a phase 2 trial in process that will study dupilumab vs placebo in adults with EGID (5). With such a lack of clinical evidence for dupilumab use in EGID, it is important to highlight a case that emphasizes the potential of dupilumab in treatment of EOG, especially in the setting of a complicated non-healing gastric ulcer.

Figure: Fig 1a: EGD showing patient's gastric ulcer, indicated by yellow arrow, upon initial discovery.
Fig 1b: EGD showing patient's gastric ulcer, indicated by yellow arrow, after 6 months of dupilumab treatment.
Fig 1b: EGD showing patient's gastric ulcer, indicated by yellow arrow, after 6 months of dupilumab treatment.
Disclosures:
Braden Thomas indicated no relevant financial relationships.
Neha Mathur indicated no relevant financial relationships.
Braden Thomas, MD, Neha Mathur, MD. P1416 - Beyond the Esophagus: Dupilumab’s Potential in Treatment of Eosinophilic Gastritis, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

