Monday Poster Session
Category: Esophagus
P1931 - A Case of Generalized Lichen Nitidus in a Patient With Eosinophilic Esophagitis on Dupilumab
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Lehar Khanna, MBBS
University of Connecticut
Hartford, CT
Presenting Author(s)
Lehar Khanna, MBBS1, Xiang Liu, MD2, Gillian Weston, MD2, Houman Rezaizadeh, MD2
1University of Connecticut, Hartford, CT; 2University of Connecticut, Farmington, CT
Introduction: Dupilumab is a human monoclonal antibody that specifically binds the IL-4 receptor and inhibits both IL-4 and IL-13 signaling. Dupilumab was recently approved by the FDA for the treatment of eosinophilic esophagitis (EoE). While cutaneous adverse effects have been reported with dupilumab, and sometimes require discontinuation or modification of therapy, we present the first case of a patient on dupilumab for EoE with generalized lichen nitidus (LN), which may not require discontinuation of dupilumab.
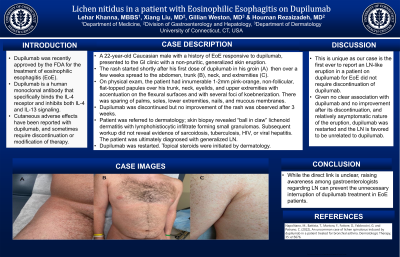
Case Description/Methods: A 22-year-old Caucasian male with a history of EoE responsive to dupilumab, presented to the GI clinic with a non-pruritic, generalized skin eruption. The rash started at the time of his first dose of dupilumab in his groin then over a few weeks spread to the abdomen, trunk, neck, and extremities. On physical exam, the patient had innumerable 1-2mm pink-orange, non-follicular, flat-topped papules over his trunk, neck, eyelids, and upper extremities with accentuation on the flexural surfaces and with several foci of koebnerization. There was sparing of palms, soles, lower extremities, nails, and mucous membranes. Dupilumab was discontinued but no improvement of the rash was observed after 3 weeks. Patient was referred to dermatology; skin biopsy revealed “ball in claw” lichenoid dermatitis with lymphohistiocytic infiltrate forming small granulomas. Subsequent workup did not reveal evidence of sarcoidosis, tuberculosis, HIV, or viral hepatitis. The patient was ultimately diagnosed with generalized LN. Given no clear association with dupilumab and no improvement after its discontinuation, and relatively asymptomatic nature of the eruption, dupilumab was restarted. Topical steroids were initiated by dermatology.
Discussion: Although there are a handful of cases with lichenoid rash possibly induced by dupilumab described in the literature, our case is the first ever to report an LN-like eruption. In this case, given the inability to confirm a temporal association between dupilumab and the development of eruption, no improvement of the eruption off the medication, the LN is favored to be unrelated to dupilumab. LN is generally considered a benign, self-limited condition and therefore, the patient is continuing dupilumab therapy for EoE and being managed symptomatically for his cutaneous eruption. While the direct link remains unclear, raising awareness among gastroenterologists regarding LN can prevent the unnecessary interruption of dupilumab treatment in EoE patients.

Disclosures:
Lehar Khanna, MBBS1, Xiang Liu, MD2, Gillian Weston, MD2, Houman Rezaizadeh, MD2. P1931 - A Case of Generalized Lichen Nitidus in a Patient With Eosinophilic Esophagitis on Dupilumab, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Connecticut, Hartford, CT; 2University of Connecticut, Farmington, CT
Introduction: Dupilumab is a human monoclonal antibody that specifically binds the IL-4 receptor and inhibits both IL-4 and IL-13 signaling. Dupilumab was recently approved by the FDA for the treatment of eosinophilic esophagitis (EoE). While cutaneous adverse effects have been reported with dupilumab, and sometimes require discontinuation or modification of therapy, we present the first case of a patient on dupilumab for EoE with generalized lichen nitidus (LN), which may not require discontinuation of dupilumab.
Case Description/Methods: A 22-year-old Caucasian male with a history of EoE responsive to dupilumab, presented to the GI clinic with a non-pruritic, generalized skin eruption. The rash started at the time of his first dose of dupilumab in his groin then over a few weeks spread to the abdomen, trunk, neck, and extremities. On physical exam, the patient had innumerable 1-2mm pink-orange, non-follicular, flat-topped papules over his trunk, neck, eyelids, and upper extremities with accentuation on the flexural surfaces and with several foci of koebnerization. There was sparing of palms, soles, lower extremities, nails, and mucous membranes. Dupilumab was discontinued but no improvement of the rash was observed after 3 weeks. Patient was referred to dermatology; skin biopsy revealed “ball in claw” lichenoid dermatitis with lymphohistiocytic infiltrate forming small granulomas. Subsequent workup did not reveal evidence of sarcoidosis, tuberculosis, HIV, or viral hepatitis. The patient was ultimately diagnosed with generalized LN. Given no clear association with dupilumab and no improvement after its discontinuation, and relatively asymptomatic nature of the eruption, dupilumab was restarted. Topical steroids were initiated by dermatology.
Discussion: Although there are a handful of cases with lichenoid rash possibly induced by dupilumab described in the literature, our case is the first ever to report an LN-like eruption. In this case, given the inability to confirm a temporal association between dupilumab and the development of eruption, no improvement of the eruption off the medication, the LN is favored to be unrelated to dupilumab. LN is generally considered a benign, self-limited condition and therefore, the patient is continuing dupilumab therapy for EoE and being managed symptomatically for his cutaneous eruption. While the direct link remains unclear, raising awareness among gastroenterologists regarding LN can prevent the unnecessary interruption of dupilumab treatment in EoE patients.

Figure: Generalized lichen nitidus eruption with shiny pink-orange papules generalized over the groin (A), chest and abdomen (B), and extremities (C).
Disclosures:
Lehar Khanna indicated no relevant financial relationships.
Xiang Liu indicated no relevant financial relationships.
Gillian Weston indicated no relevant financial relationships.
Houman Rezaizadeh: AstraZeneca – Stock-publicly held company(excluding mutual/index funds). Regeneron – Advisory Committee/Board Member, Speakers Bureau. Sanofi – Advisory Committee/Board Member. Skope Inc – Consultant, Stock Options, Stock-privately held company.
Lehar Khanna, MBBS1, Xiang Liu, MD2, Gillian Weston, MD2, Houman Rezaizadeh, MD2. P1931 - A Case of Generalized Lichen Nitidus in a Patient With Eosinophilic Esophagitis on Dupilumab, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
