Monday Poster Session
Category: Functional Bowel Disease
P1959 - Efficacy and Safety of Prucalopride in Patients With Chronic Idiopathic Constipation From Different Racial and Ethnic Groups: A Post Hoc Analysis of Phase 3 and 4 Clinical Study Data
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- AL
Anthony Lembo, MD
Digestive Disease and Surgery Institute, Cleveland Clinic
Cleveland, Ohio
Presenting Author(s)
Anthony Lembo, MD1, Kyle Staller, MD, MPH2, Brian Terreri, PharmD, MBA3, Brooks D Cash, MD, FACG4, Paul Feuerstadt, MD, FACG5, André Gabriel, MS, MD6, William Spalding, MS7, Yaping Wan, MS6, Yunlong Xie, PhD6, Ashraf Youssef, MD, PhD, MBA8, Mena Boules, MD3
1Digestive Disease and Surgery Institute, Cleveland Clinic, Cleveland, OH; 2Massachusetts General Hospital, Boston, MA; 3Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA; 4University of Texas Health Science Center at Houston, Houston, TX; 5PACT Gastroenterology Center and Yale School of Medicine, Hamden, CT; 6Takeda Development Center Americas, Inc., Cambridge, MA; 7Takeda Development Center Americas, Inc., Lexington, MA; 8Takeda Pharmaceuticals U.S.A., Inc., Cambridge, MA
Introduction: Although racial and ethnic minority groups are disproportionately affected by constipation in the USA, they are underrepresented in clinical studies. This post hoc analysis aimed to evaluate the efficacy and safety of prucalopride in patients with chronic idiopathic constipation (CIC) stratified by different racial and ethnic groups.
Methods: Data from six key phase 3–4 randomized, double-blind, placebo-controlled studies of prucalopride (1 or 2 mg once daily) over 12 weeks were pooled. Patients with CIC were stratified by physician-reported race or ethnicity (Asian, Black or African American, Hispanic, White, other and missing information). The prespecified primary endpoint was the proportion of patients with a mean frequency of ≥3 complete spontaneous bowel movements per week over weeks 1–12. Safety data were evaluated descriptively.
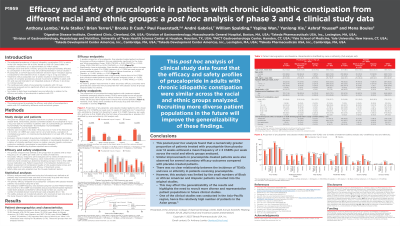
Results: Of the 2484 patients included in the full analysis set (mean age, 47.4 years; 76.0% female), 478 (19.2%) were Asian, 78 (3.1%) were Black or African American, 19 (0.8%) were Hispanic and 1857 (74.8%) were White. Patient demographics and baseline characteristics are shown in the Table. A total of 34 patients (1.4%) reported their race or ethnicity as ‘other’; for 18 patients (0.7%) this information was missing. These two groups were not analyzed further. Within each racial and ethnic group, a numerically greater proportion of prucalopride-treated patients achieved the primary efficacy endpoint compared with placebo (Asian: 32.8% vs 10.5%; Black or African American: 17.4% vs 9.4%; Hispanic: 12.5% vs 0.0%; White: 27.8% vs 14.3%; Fig. A). The proportions of prucalopride-treated patients with treatment-related treatment-emergent adverse events (TEAEs) were similar across all racial and ethnic groups (Asian: 36.1%; Black or African American: 37.0%; Hispanic: 37.5%; White: 36.3%) and were higher than for placebo (Fig. B). However, most TEAEs were unrelated to the study drug and were mild or moderate in severity across each racial and ethnic group analyzed (Fig. B).
Discussion: Efficacy and safety outcomes for prucalopride in patients with CIC were similar across the racial and ethnic groups analyzed; however, this analysis was limited by the small numbers of Black or African American and Hispanic patients recruited into the original studies. This may affect the generalizability of the results and highlights the need to recruit more diverse and representative patient populations in future clinical studies.

Disclosures:
Anthony Lembo, MD1, Kyle Staller, MD, MPH2, Brian Terreri, PharmD, MBA3, Brooks D Cash, MD, FACG4, Paul Feuerstadt, MD, FACG5, André Gabriel, MS, MD6, William Spalding, MS7, Yaping Wan, MS6, Yunlong Xie, PhD6, Ashraf Youssef, MD, PhD, MBA8, Mena Boules, MD3. P1959 - Efficacy and Safety of Prucalopride in Patients With Chronic Idiopathic Constipation From Different Racial and Ethnic Groups: A Post Hoc Analysis of Phase 3 and 4 Clinical Study Data, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Digestive Disease and Surgery Institute, Cleveland Clinic, Cleveland, OH; 2Massachusetts General Hospital, Boston, MA; 3Takeda Pharmaceuticals U.S.A., Inc., Lexington, MA; 4University of Texas Health Science Center at Houston, Houston, TX; 5PACT Gastroenterology Center and Yale School of Medicine, Hamden, CT; 6Takeda Development Center Americas, Inc., Cambridge, MA; 7Takeda Development Center Americas, Inc., Lexington, MA; 8Takeda Pharmaceuticals U.S.A., Inc., Cambridge, MA
Introduction: Although racial and ethnic minority groups are disproportionately affected by constipation in the USA, they are underrepresented in clinical studies. This post hoc analysis aimed to evaluate the efficacy and safety of prucalopride in patients with chronic idiopathic constipation (CIC) stratified by different racial and ethnic groups.
Methods: Data from six key phase 3–4 randomized, double-blind, placebo-controlled studies of prucalopride (1 or 2 mg once daily) over 12 weeks were pooled. Patients with CIC were stratified by physician-reported race or ethnicity (Asian, Black or African American, Hispanic, White, other and missing information). The prespecified primary endpoint was the proportion of patients with a mean frequency of ≥3 complete spontaneous bowel movements per week over weeks 1–12. Safety data were evaluated descriptively.
Results: Of the 2484 patients included in the full analysis set (mean age, 47.4 years; 76.0% female), 478 (19.2%) were Asian, 78 (3.1%) were Black or African American, 19 (0.8%) were Hispanic and 1857 (74.8%) were White. Patient demographics and baseline characteristics are shown in the Table. A total of 34 patients (1.4%) reported their race or ethnicity as ‘other’; for 18 patients (0.7%) this information was missing. These two groups were not analyzed further. Within each racial and ethnic group, a numerically greater proportion of prucalopride-treated patients achieved the primary efficacy endpoint compared with placebo (Asian: 32.8% vs 10.5%; Black or African American: 17.4% vs 9.4%; Hispanic: 12.5% vs 0.0%; White: 27.8% vs 14.3%; Fig. A). The proportions of prucalopride-treated patients with treatment-related treatment-emergent adverse events (TEAEs) were similar across all racial and ethnic groups (Asian: 36.1%; Black or African American: 37.0%; Hispanic: 37.5%; White: 36.3%) and were higher than for placebo (Fig. B). However, most TEAEs were unrelated to the study drug and were mild or moderate in severity across each racial and ethnic group analyzed (Fig. B).
Discussion: Efficacy and safety outcomes for prucalopride in patients with CIC were similar across the racial and ethnic groups analyzed; however, this analysis was limited by the small numbers of Black or African American and Hispanic patients recruited into the original studies. This may affect the generalizability of the results and highlights the need to recruit more diverse and representative patient populations in future clinical studies.

Figure: Figure. The proportion of patients with (A) a mean frequency of ≥3 CSBMs per week over weeks 1–12^a (full analysis set)^b and (B) TEAEs (safety analysis set)^c, stratified by race and ethnicity.
The full analysis set included all patients who received at least one dose of the study drug and had at least one efficacy assessment after receiving a dose.
The safety analysis set included all patients who received at least one dose of the study drug.
The 34 patients who reported their race and ethnicity as 'other' and the 18 patients for whom this information was missing were not included in this analysis. A breakdown of the racial and ethnic groups included in the ‘other’ category is not available.
a^p values were obtained using the χ2 test, except for the Hispanic group, for which the p value was obtained using Fisher’s exact test.
b^For prucalopride 1 or 2 mg once daily: n=241 (Asian), n=46 (Black or African American), n=8 (Hispanic), n=915 (White); for placebo: n=237 (Asian), n=32 (Black or African American), n=11 (Hispanic), n=940 (White).
c^For prucalopride 1 or 2 mg once daily: n=241 (Asian), n=46 (Black or African American), n=8 (Hispanic), n=953 (White); for placebo: n=237 (Asian), n=32 (Black or African American), n=11 (Hispanic), n=972 (White).
CSBM, complete spontaneous bowel movement; TEAE, treatment-emergent adverse event.
The full analysis set included all patients who received at least one dose of the study drug and had at least one efficacy assessment after receiving a dose.
The safety analysis set included all patients who received at least one dose of the study drug.
The 34 patients who reported their race and ethnicity as 'other' and the 18 patients for whom this information was missing were not included in this analysis. A breakdown of the racial and ethnic groups included in the ‘other’ category is not available.
a^p values were obtained using the χ2 test, except for the Hispanic group, for which the p value was obtained using Fisher’s exact test.
b^For prucalopride 1 or 2 mg once daily: n=241 (Asian), n=46 (Black or African American), n=8 (Hispanic), n=915 (White); for placebo: n=237 (Asian), n=32 (Black or African American), n=11 (Hispanic), n=940 (White).
c^For prucalopride 1 or 2 mg once daily: n=241 (Asian), n=46 (Black or African American), n=8 (Hispanic), n=953 (White); for placebo: n=237 (Asian), n=32 (Black or African American), n=11 (Hispanic), n=972 (White).
CSBM, complete spontaneous bowel movement; TEAE, treatment-emergent adverse event.
Disclosures:
Anthony Lembo: AEON Biopharma Inc. – Consultant. Alkermes – Consultant. Allakos – Consultant. Allurion – Stock Options. Anji Pharmaceuticals – Consultant. Arena Pharmaceuticals – Consultant. BioAmerica – Consultant. Bristol Myers Squibb – Stock Options. Gemelli Biotech – Consultant. Ironwood Pharmaceuticals – Consultant. Johnson & Johnson – Stock Options. Maunea Kea – Consultant. Neurogastrx, Inc. – Consultant. OrphoMed, Inc. – Consultant. Pfizer – Consultant. QOL Medical – Consultant. Shire, a Takeda company – Consultant. Takeda Pharmaceuticals – Consultant. Vibrant Pharma, Inc. – Consultant.
Kyle Staller: Anji Pharmaceuticals – Consultant. Ardelyx – Consultant. Gelesis – Consultant. GI Supply, a Laborie company – Consultant. Ironwood Pharmaceuticals – Grant/Research Support. ReStalsis Health – Consultant. Sanofi – Consultant. Urovant Sciences – Grant/Research Support.
Brian Terreri: Takeda Pharmaceutical Company Limited – Stock-privately held company. Takeda Pharmaceuticals USA, Inc. – Employee.
Brooks D Cash: AbbVie – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. QOL Medical – Consultant, Speakers Bureau. Salix Pharmaceuticals – Consultant, Speakers Bureau. Takeda Pharmaceuticals – Consultant, Speakers Bureau.
Paul Feuerstadt: Ferring/Rebiotix, Inc. – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck & Co. – Consultant, Speakers Bureau. Seres Therapeutics – Advisory Committee/Board Member, Consultant, Speakers Bureau. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau.
André Gabriel: Takeda Development Center Americas, Inc. – Employee. Takeda Pharmaceutical Company Limited – Stock-privately held company.
William Spalding: Takeda Development Center Americas, Inc, – Employee. Takeda Pharmaceutical Company Limited – Stock-privately held company.
Yaping Wan: Takeda Development Center Americas, Inc. – Employee. Takeda Pharmaceutical Company Limited – Stock-privately held company.
Yunlong Xie: Takeda Development Center Americas, Inc. – Employee. Takeda Pharmaceutical Company Limited – Stock-privately held company.
Ashraf Youssef: Takeda Pharmaceutical Company Limited – Stock-privately held company. Takeda Pharmaceuticals USA, Inc. – Employee.
Mena Boules: Takeda Pharmaceutical Company Limited – Stock-privately held company. Takeda Pharmaceuticals USA, Inc. – Employee.
Anthony Lembo, MD1, Kyle Staller, MD, MPH2, Brian Terreri, PharmD, MBA3, Brooks D Cash, MD, FACG4, Paul Feuerstadt, MD, FACG5, André Gabriel, MS, MD6, William Spalding, MS7, Yaping Wan, MS6, Yunlong Xie, PhD6, Ashraf Youssef, MD, PhD, MBA8, Mena Boules, MD3. P1959 - Efficacy and Safety of Prucalopride in Patients With Chronic Idiopathic Constipation From Different Racial and Ethnic Groups: A Post Hoc Analysis of Phase 3 and 4 Clinical Study Data, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
