Monday Poster Session
Category: General Endoscopy
P1984 - Safety and Efficacy of Diphenhydramine as an Adjunct to Midazolam and Fentanyl for Sedation in Patients Under the Age of 45 Undergoing Endoscopic Evaluation
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Danyal O. Imam, MD
Riverside University Health System
Moreno Valley, CA
Presenting Author(s)
Danyal O. Imam, MD1, Yinglin Gao, DO2, Timothy Allison-Aipa, PhD3, Manish Shrestha, MD1, Steve Serrao, MD, PhD1, Wichit Srikureja, MD1, Nikhil Thiruvengadam, MD4, Pejman Solaimani, MD4
1Riverside University Health System, Moreno Valley, CA; 2Loma Linda University Health, Loma Linda, CA; 3Riverside University Health System, Comparative Effectiveness and Clinical Outcomes Research Center, Moreno Valley, CA; 4Loma Linda University, Loma Linda, CA
Introduction: Midazolam and fentanyl are commonly used for conscious sedation during endoscopic procedures. Achieving adequate sedation can be difficult in younger patients (pts). As a result, these pts are often referred for anesthesiologist-administered sedation. Prior studies have shown that diphenhydramine (DPH) as an adjunctive sedative in colonoscopy is safe but data in pts under the age of 45 is lacking. This study aims to evaluate the efficacy and safety of DPH as an adjunct to fentanyl and midazolam during endoscopy in this pt population.
Methods: A retrospective, single-center cohort study was performed in pts aged 18-44 years who underwent esophagogastroduodenoscopy (EGD), colonoscopy or both while receiving midazolam and fentanyl for conscious sedation with or without DPH between 2020 and 2021 at a county hospital. Pts who received DPH as an adjunctive sedative (DPH group) were compared with those who did not (non-DPH group). Outcomes included rates of adverse events, total facility time, total dose of midazolam and fentanyl, total recovery time and total time to adequate sedation. A chi-square test was used to compare the incidence of adverse events. An independent samples T-test was used to compare the total facility time, recovery time, time to adequate sedation and amount of midazolam and fentanyl used.
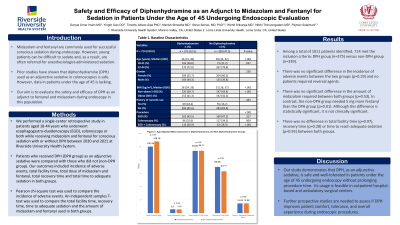
Results: Among a total of 1011 pts identified, 714 met the inclusion criteria: DPH group (n=375) versus non-DPH group (n=339). There was no significant difference in narcotic use amongst the two groups (p=0.443). Non-DPH group were more likely to be obese than DPH group (p< 0.001). There was no significant difference in the incidence of adverse events between the two groups (p=0.29) and no pts required reversal agents. Furthermore, there was no difference in total facility time (p=0.97), recovery time (p=0.28) or time to reach adequate sedation (p=0.95) between two groups. There was no significant difference in the amount of midazolam required (p=0.53), however, non-DPH group needed significantly more fentanyl than DPH group (p=0.01).
Discussion: Our study demonstrates that DPH as an adjunctive sedative is safe and well-tolerated in pts under age 45 undergoing endoscopy. DPH usage did not prolong procedure time suggesting its usage is feasible in outpatient hospital-based and ambulatory surgery centers. Further prospective studies are needed to assess if DPH improves pt comfort, tolerance, and overall experience during endoscopic procedures.

Disclosures:
Danyal O. Imam, MD1, Yinglin Gao, DO2, Timothy Allison-Aipa, PhD3, Manish Shrestha, MD1, Steve Serrao, MD, PhD1, Wichit Srikureja, MD1, Nikhil Thiruvengadam, MD4, Pejman Solaimani, MD4. P1984 - Safety and Efficacy of Diphenhydramine as an Adjunct to Midazolam and Fentanyl for Sedation in Patients Under the Age of 45 Undergoing Endoscopic Evaluation, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Riverside University Health System, Moreno Valley, CA; 2Loma Linda University Health, Loma Linda, CA; 3Riverside University Health System, Comparative Effectiveness and Clinical Outcomes Research Center, Moreno Valley, CA; 4Loma Linda University, Loma Linda, CA
Introduction: Midazolam and fentanyl are commonly used for conscious sedation during endoscopic procedures. Achieving adequate sedation can be difficult in younger patients (pts). As a result, these pts are often referred for anesthesiologist-administered sedation. Prior studies have shown that diphenhydramine (DPH) as an adjunctive sedative in colonoscopy is safe but data in pts under the age of 45 is lacking. This study aims to evaluate the efficacy and safety of DPH as an adjunct to fentanyl and midazolam during endoscopy in this pt population.
Methods: A retrospective, single-center cohort study was performed in pts aged 18-44 years who underwent esophagogastroduodenoscopy (EGD), colonoscopy or both while receiving midazolam and fentanyl for conscious sedation with or without DPH between 2020 and 2021 at a county hospital. Pts who received DPH as an adjunctive sedative (DPH group) were compared with those who did not (non-DPH group). Outcomes included rates of adverse events, total facility time, total dose of midazolam and fentanyl, total recovery time and total time to adequate sedation. A chi-square test was used to compare the incidence of adverse events. An independent samples T-test was used to compare the total facility time, recovery time, time to adequate sedation and amount of midazolam and fentanyl used.
Results: Among a total of 1011 pts identified, 714 met the inclusion criteria: DPH group (n=375) versus non-DPH group (n=339). There was no significant difference in narcotic use amongst the two groups (p=0.443). Non-DPH group were more likely to be obese than DPH group (p< 0.001). There was no significant difference in the incidence of adverse events between the two groups (p=0.29) and no pts required reversal agents. Furthermore, there was no difference in total facility time (p=0.97), recovery time (p=0.28) or time to reach adequate sedation (p=0.95) between two groups. There was no significant difference in the amount of midazolam required (p=0.53), however, non-DPH group needed significantly more fentanyl than DPH group (p=0.01).
Discussion: Our study demonstrates that DPH as an adjunctive sedative is safe and well-tolerated in pts under age 45 undergoing endoscopy. DPH usage did not prolong procedure time suggesting its usage is feasible in outpatient hospital-based and ambulatory surgery centers. Further prospective studies are needed to assess if DPH improves pt comfort, tolerance, and overall experience during endoscopic procedures.

Figure: Age Adjusted Mean Outcomes in Diphenhydramine and Non-Diphenhydramine Groups
Disclosures:
Danyal Imam indicated no relevant financial relationships.
Yinglin Gao indicated no relevant financial relationships.
Timothy Allison-Aipa indicated no relevant financial relationships.
Manish Shrestha indicated no relevant financial relationships.
Steve Serrao indicated no relevant financial relationships.
Wichit Srikureja indicated no relevant financial relationships.
Nikhil Thiruvengadam indicated no relevant financial relationships.
Pejman Solaimani indicated no relevant financial relationships.
Danyal O. Imam, MD1, Yinglin Gao, DO2, Timothy Allison-Aipa, PhD3, Manish Shrestha, MD1, Steve Serrao, MD, PhD1, Wichit Srikureja, MD1, Nikhil Thiruvengadam, MD4, Pejman Solaimani, MD4. P1984 - Safety and Efficacy of Diphenhydramine as an Adjunct to Midazolam and Fentanyl for Sedation in Patients Under the Age of 45 Undergoing Endoscopic Evaluation, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
