Monday Poster Session
Category: GI Bleeding
P2046 - The Clot Thickens - Hemostatic Powder as Primary Therapy in Non-variceal Upper Gastrointestinal Bleeding: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
.jpg)
Dalton A. Norwood, MD
UAB Minority Health and Health Equity Research Center, The University of Alabama at Birmingham Heersink School of Medicine
Birmingham, AL
Presenting Author(s)
Award: Outstanding Research Award in the GI Bleeding Category
Award: Presidential Poster Award
Dalton A. Norwood, MD1, Eleazar E.. Montalvan-Sanchez, MD2, Chad Burski, MD3, Ramzi Mulki, MD4, Sergio A. Sánchez-Luna, MD4, Ali Mir Ahmed, MD3, Kondal Kyanam, MD4, Shajan Peter, MD3
1UAB Minority Health and Health Equity Research Center, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Alabama at Birmingham, Birmingham, AL; 4Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL
Introduction: Hemostatic powder (HP) has emerged as a potential therapeutic option in non-variceal upper gastrointestinal bleeding. However, the evidence regarding its efficacy and safety as primary therapy remains inconclusive. We aimed to evaluate the effectiveness of hemostatic powder in achieving primary hemostasis, reducing rebleeding rates, minimizing mortality, and assessing its impact on length of hospital stay (LOS) in patients with non-variceal upper gastrointestinal bleeding in a meta-analysis
Methods: We used the PubMed Scopus and Web of Science databases, through June 2023 to identify RCTs assessing the efficacy of HP compared with Standard of care (Hemoclip, thermocoagulation and epinephrine injection). We followed the (PRISMA) protocol. Two independent reviewers assessed study quality/risk for bias using Newcastle-Ottawa Scale (NOS) and extracted descriptive and quantitative data. Meta-analysis was performed using appropriate statistical methods to estimate pooled effect sizes, risk ratios, and heterogeneity.
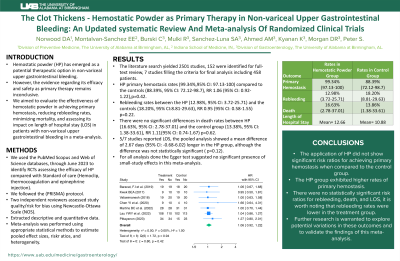
Results: The literature search yielded 2501 studies, of which 152 were identified for full-text review, with 7 studies filling the criteria for final analysis including 458 patients. HP primary hemostasis rates (99.34%, 95% CI: 97.13-100) compared to the controls (88.39%, 95% CI: 72.12-98.7), RR 1.06 (95% CI: 0.92-1.22), p=0.42. Rebleeding rates between the HP (12.98%, 95% CI: 3.72-25.71) and the controls (18.20%, 95% CI: 8.81-29.63), RR 0.95 (95% CI: 0.58-1.55) p=0.22. There were no significant differences in death rates between HP (16.63%, 95% CI: 2.78-37.01) and the control group (13.38%, 95% CI: 1.38-33.61), RR 1.11 (95% CI: 0.74-1.67) p=0.62. 5/7 studies reported LOS, the pooled analysis showed a mean difference of 2.67 days (95% CI: -0.68-6.02) longer in the HP group, although the difference was not statistically significant ( p=0.12). For all analysis done the Egger test suggested no significant presence of small-study effects in this meta-analysis.
Discussion: The application of HP did not show significant risk ratios for achieving primary hemostasis when compared to the control group. Nevertheless, the HP group exhibited higher rates of primary hemostasis. There were no statistically significant risk ratios for rebleeding, death, and LOS, it is worth noting that rebleeding rates were lower in the treatment group. Further research is warranted to explore potential variations in these outcomes and to validate the findings of this meta-analysis

Disclosures:
Dalton A. Norwood, MD1, Eleazar E.. Montalvan-Sanchez, MD2, Chad Burski, MD3, Ramzi Mulki, MD4, Sergio A. Sánchez-Luna, MD4, Ali Mir Ahmed, MD3, Kondal Kyanam, MD4, Shajan Peter, MD3. P2046 - The Clot Thickens - Hemostatic Powder as Primary Therapy in Non-variceal Upper Gastrointestinal Bleeding: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Award: Presidential Poster Award
Dalton A. Norwood, MD1, Eleazar E.. Montalvan-Sanchez, MD2, Chad Burski, MD3, Ramzi Mulki, MD4, Sergio A. Sánchez-Luna, MD4, Ali Mir Ahmed, MD3, Kondal Kyanam, MD4, Shajan Peter, MD3
1UAB Minority Health and Health Equity Research Center, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Alabama at Birmingham, Birmingham, AL; 4Basil I. Hirschowitz Endoscopic Center of Excellence, The University of Alabama at Birmingham Heersink School of Medicine, Birmingham, AL
Introduction: Hemostatic powder (HP) has emerged as a potential therapeutic option in non-variceal upper gastrointestinal bleeding. However, the evidence regarding its efficacy and safety as primary therapy remains inconclusive. We aimed to evaluate the effectiveness of hemostatic powder in achieving primary hemostasis, reducing rebleeding rates, minimizing mortality, and assessing its impact on length of hospital stay (LOS) in patients with non-variceal upper gastrointestinal bleeding in a meta-analysis
Methods: We used the PubMed Scopus and Web of Science databases, through June 2023 to identify RCTs assessing the efficacy of HP compared with Standard of care (Hemoclip, thermocoagulation and epinephrine injection). We followed the (PRISMA) protocol. Two independent reviewers assessed study quality/risk for bias using Newcastle-Ottawa Scale (NOS) and extracted descriptive and quantitative data. Meta-analysis was performed using appropriate statistical methods to estimate pooled effect sizes, risk ratios, and heterogeneity.
Results: The literature search yielded 2501 studies, of which 152 were identified for full-text review, with 7 studies filling the criteria for final analysis including 458 patients. HP primary hemostasis rates (99.34%, 95% CI: 97.13-100) compared to the controls (88.39%, 95% CI: 72.12-98.7), RR 1.06 (95% CI: 0.92-1.22), p=0.42. Rebleeding rates between the HP (12.98%, 95% CI: 3.72-25.71) and the controls (18.20%, 95% CI: 8.81-29.63), RR 0.95 (95% CI: 0.58-1.55) p=0.22. There were no significant differences in death rates between HP (16.63%, 95% CI: 2.78-37.01) and the control group (13.38%, 95% CI: 1.38-33.61), RR 1.11 (95% CI: 0.74-1.67) p=0.62. 5/7 studies reported LOS, the pooled analysis showed a mean difference of 2.67 days (95% CI: -0.68-6.02) longer in the HP group, although the difference was not statistically significant ( p=0.12). For all analysis done the Egger test suggested no significant presence of small-study effects in this meta-analysis.
Discussion: The application of HP did not show significant risk ratios for achieving primary hemostasis when compared to the control group. Nevertheless, the HP group exhibited higher rates of primary hemostasis. There were no statistically significant risk ratios for rebleeding, death, and LOS, it is worth noting that rebleeding rates were lower in the treatment group. Further research is warranted to explore potential variations in these outcomes and to validate the findings of this meta-analysis

Figure: Primary hemostasis with Hemostatic powder - Hazard rations
Disclosures:
Dalton Norwood indicated no relevant financial relationships.
Eleazar Montalvan-Sanchez indicated no relevant financial relationships.
Chad Burski: astrazeneca – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Kimberly-Clark Corporation – Stock-publicly held company(excluding mutual/index funds). merck – Stock-publicly held company(excluding mutual/index funds). Proctor and Gamble – Stock-publicly held company(excluding mutual/index funds).
Ramzi Mulki indicated no relevant financial relationships.
Sergio A. Sánchez-Luna: ASGE - Fujifilm Research Grant Award – Grant/Research Support.
Ali Mir Ahmed indicated no relevant financial relationships.
Kondal Kyanam indicated no relevant financial relationships.
Shajan Peter indicated no relevant financial relationships.
Dalton A. Norwood, MD1, Eleazar E.. Montalvan-Sanchez, MD2, Chad Burski, MD3, Ramzi Mulki, MD4, Sergio A. Sánchez-Luna, MD4, Ali Mir Ahmed, MD3, Kondal Kyanam, MD4, Shajan Peter, MD3. P2046 - The Clot Thickens - Hemostatic Powder as Primary Therapy in Non-variceal Upper Gastrointestinal Bleeding: An Updated Systematic Review and Meta-Analysis of Randomized Clinical Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

