Monday Poster Session
Category: IBD
P2124 - A New Evaluation Method to Confirm Histological Mucosal Healing of Ulcerative Colitis On-Site Without Biopsy by Checking Biomarkers Endoscopically
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Hidetoshi Ohta, MD, PhD
Sapporo Orthopaedics and Cardiovascular Hospital
Sapporo, Hokkaido, Japan
Presenting Author(s)
Hidetoshi Ohta, MD, PhD
Sapporo Orthopaedics and Cardiovascular Hospital, Sapporo, Hokkaido, Japan
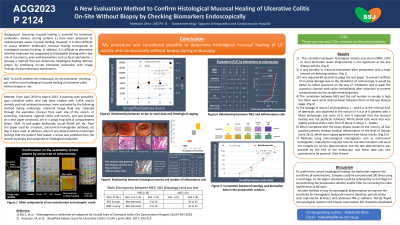
Introduction: Assessing mucosal healing is essential for treatment evaluation. Various scoring systems 1] have been proposed to endoscopically assess mucosal healing. However, it is often difficult to assess whether endoscopic mucosal healing corresponds to histological mucosal healing. In addition, it is difficult to determine whether treatment has progressed to histological healing with a low risk of recurrence, even with biomarkers such as fecal calprotectin. I devised a method that can confirm histological healing without biopsy by checking biomarkers on-site.
Methods: From April 2019 to March 2022, 8 patients with pancolitis-type ulcerative colitis who had been treated with 5-ASA and/or steroids and had achieved remission. Preparation fluid was excreted through the aspiration channel from each site of ascending, transverse, sigmoid colon, and rectum, and was sprayed over test papers set in a polyp trap with 8 compartments (Ezem, USA). Test papers for detecting leukocytes, occult blood, pH, etc. on urinalysis or fecal occult blood were used. In addition, when endoscopic findings shew mucosal healing, tissue samples were collected from the rectum by biopsy and subjected to histological evaluations.
Results: 1) It was possible to measure biomarkers after preparation with a large amount of cleansing solution. 2) One minutes were required to evaluate biomarkers while performing endoscopy. 3) To prevent artifacts due to the manipulation of scope, it would be better to collect fluid while inserting colonoscope. 4) I needed to cleanse the accessory channel immediately after collection to avoid contamination by the remained sample. 5) A few of erythrocytes and leukocytes were detected in the rectum in 4 out of 8 patients with Mayo endoscopic sub score of 0, and it indicated that their mucosal healings were not perfectly complete. 6) When compared with the tissue biopsy results of the rectum, all 4 positive patients showed residual inflammation of Geboes Score 2B 2], which was in good agreement with my test. 7) Immunological assay methods such as FOB, Calprotectin required time for judgement and were not suitable for on-site determination.
Discussion: My procedure was considered possible to determine histological mucosal healing simply and noninvasively without biopsy during endoscopy.
1] Ma C, et al. Heterogeneity in definition… Clin Gastroenterol Hepatol 16:637-647,2018
2] Aranzazu J A, et al. Simplified Geboes Score J Crohn’s and Colitis, 305-313,2017

Disclosures:
Hidetoshi Ohta, MD, PhD. P2124 - A New Evaluation Method to Confirm Histological Mucosal Healing of Ulcerative Colitis On-Site Without Biopsy by Checking Biomarkers Endoscopically, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Sapporo Orthopaedics and Cardiovascular Hospital, Sapporo, Hokkaido, Japan
Introduction: Assessing mucosal healing is essential for treatment evaluation. Various scoring systems 1] have been proposed to endoscopically assess mucosal healing. However, it is often difficult to assess whether endoscopic mucosal healing corresponds to histological mucosal healing. In addition, it is difficult to determine whether treatment has progressed to histological healing with a low risk of recurrence, even with biomarkers such as fecal calprotectin. I devised a method that can confirm histological healing without biopsy by checking biomarkers on-site.
Methods: From April 2019 to March 2022, 8 patients with pancolitis-type ulcerative colitis who had been treated with 5-ASA and/or steroids and had achieved remission. Preparation fluid was excreted through the aspiration channel from each site of ascending, transverse, sigmoid colon, and rectum, and was sprayed over test papers set in a polyp trap with 8 compartments (Ezem, USA). Test papers for detecting leukocytes, occult blood, pH, etc. on urinalysis or fecal occult blood were used. In addition, when endoscopic findings shew mucosal healing, tissue samples were collected from the rectum by biopsy and subjected to histological evaluations.
Results: 1) It was possible to measure biomarkers after preparation with a large amount of cleansing solution. 2) One minutes were required to evaluate biomarkers while performing endoscopy. 3) To prevent artifacts due to the manipulation of scope, it would be better to collect fluid while inserting colonoscope. 4) I needed to cleanse the accessory channel immediately after collection to avoid contamination by the remained sample. 5) A few of erythrocytes and leukocytes were detected in the rectum in 4 out of 8 patients with Mayo endoscopic sub score of 0, and it indicated that their mucosal healings were not perfectly complete. 6) When compared with the tissue biopsy results of the rectum, all 4 positive patients showed residual inflammation of Geboes Score 2B 2], which was in good agreement with my test. 7) Immunological assay methods such as FOB, Calprotectin required time for judgement and were not suitable for on-site determination.
Discussion: My procedure was considered possible to determine histological mucosal healing simply and noninvasively without biopsy during endoscopy.
1] Ma C, et al. Heterogeneity in definition… Clin Gastroenterol Hepatol 16:637-647,2018
2] Aranzazu J A, et al. Simplified Geboes Score J Crohn’s and Colitis, 305-313,2017

Figure: Endoscopic biomarker check
Disclosures:
Hidetoshi Ohta indicated no relevant financial relationships.
Hidetoshi Ohta, MD, PhD. P2124 - A New Evaluation Method to Confirm Histological Mucosal Healing of Ulcerative Colitis On-Site Without Biopsy by Checking Biomarkers Endoscopically, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
