Monday Poster Session
Category: IBD
P2145 - Comparison of Upadacitinib Induction in Tofacitinib Exposed and Naïve Patients With Inflammatory Bowel Disease: A Real World Multi-Center Study
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Hisham S. Almomen, MRCP
Yale University School of Medicine
New Haven, CT
Presenting Author(s)
Hisham S. Almomen, MRCP1, Ibrahim Alomar, MBBS2, Chandler McMillan, BA1, Jill Gaidos, MD, FACG3, Deborah D. Proctor, MD4, Abdulelah AlMutairdi, MD2, Badr Al-Bawardy, MD2
1Yale University School of Medicine, New Haven, CT; 2King Faisal Specialist Hospital and Research Center, Riyadh, Ar Riyad, Saudi Arabia; 3Yale University, New Haven, CT; 4Yale University, Durham, CT
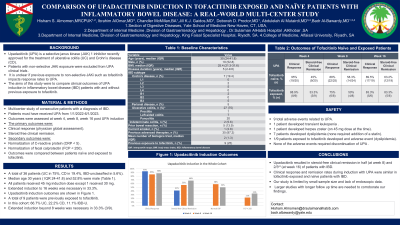
Introduction: Upadacitinib (UPA) is a selective janus kinase (JAK) 1 inhibitor recently approved for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD). It is unclear if previous exposure to non-selective JAK inhibitors such as tofacitinib impacts response rates to upadacitinib. The aim of this study is to compare clinical outcomes of UPA induction in inflammatory bowel disease (IBD) patients with and without previous exposure to tofacitinib
Methods: This was a multicenter study of consecutive patients with a diagnosis of inflammatory bowel disease who received UPA from 1/1/2022-6/1/2023. Data was abstracted for baseline demographics, disease location/phenotype, previous medication exposures, UPA dosing and duration. Outcomes were assessed at week 4, week 8 and week 16 post initiation of UPA. The primary outcomes were clinical response and steroid free clinical remission as evaluated by physician global assessment. Secondary outcomes included normalization of C-reactive protein (CRP < 5) and fecal calprotectin response (FCP < 250).
Results: A total of 36 patients [median age 30 years (interquartile range (IQR) 24-41.8); 52.8% were male]. UPA was utilized for UC in 75% (n=27), CD in 19.4% (n=7) and IBD-unclassified in 5.6% (n=2) (Table 1). All patients received the 45 mg induction dose except 1 patient received 30 mg. Extended induction to 16 weeks was necessary in 33.3% (n=12). In the entire cohort, week 8 outcomes of clinical response were achieved in 84.9% (28/33), steroid free clinical remission in 56.3% (18/32), normal CRP in 58.3% (7/12) and FCP response in 22.2% (2/9).
A total of 9 patients were previously exposed to tofacitinib (66.7% UC, 22.2% CD, 11.1% IBD-U; 44.4% were male). Extended induction beyond 8 weeks was necessary in 33.3% (3/9). Week 8 outcomes of clinical response were achieved in 75% (6/8), steroid free clinical remission in 50% (4/8). At week 16, 83.3% (5/6) of these patients achieved steroid free clinical remission. Table 2
Discussion: Upadacitinib resulted in steroid-free clinical remission in half (at week 8) and more than three-quarters (at week 16) of patients with IBD and previous exposure to tofacitinib. Clinical response and remission rates during induction with UPA were similar in tofacitinib exposed and naïve patients. Larger studies are needed to corroborate our findings.
Disclosures:
Hisham S. Almomen, MRCP1, Ibrahim Alomar, MBBS2, Chandler McMillan, BA1, Jill Gaidos, MD, FACG3, Deborah D. Proctor, MD4, Abdulelah AlMutairdi, MD2, Badr Al-Bawardy, MD2. P2145 - Comparison of Upadacitinib Induction in Tofacitinib Exposed and Naïve Patients With Inflammatory Bowel Disease: A Real World Multi-Center Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Yale University School of Medicine, New Haven, CT; 2King Faisal Specialist Hospital and Research Center, Riyadh, Ar Riyad, Saudi Arabia; 3Yale University, New Haven, CT; 4Yale University, Durham, CT
Introduction: Upadacitinib (UPA) is a selective janus kinase (JAK) 1 inhibitor recently approved for the treatment of ulcerative colitis (UC) and Crohn’s disease (CD). It is unclear if previous exposure to non-selective JAK inhibitors such as tofacitinib impacts response rates to upadacitinib. The aim of this study is to compare clinical outcomes of UPA induction in inflammatory bowel disease (IBD) patients with and without previous exposure to tofacitinib
Methods: This was a multicenter study of consecutive patients with a diagnosis of inflammatory bowel disease who received UPA from 1/1/2022-6/1/2023. Data was abstracted for baseline demographics, disease location/phenotype, previous medication exposures, UPA dosing and duration. Outcomes were assessed at week 4, week 8 and week 16 post initiation of UPA. The primary outcomes were clinical response and steroid free clinical remission as evaluated by physician global assessment. Secondary outcomes included normalization of C-reactive protein (CRP < 5) and fecal calprotectin response (FCP < 250).
Results: A total of 36 patients [median age 30 years (interquartile range (IQR) 24-41.8); 52.8% were male]. UPA was utilized for UC in 75% (n=27), CD in 19.4% (n=7) and IBD-unclassified in 5.6% (n=2) (Table 1). All patients received the 45 mg induction dose except 1 patient received 30 mg. Extended induction to 16 weeks was necessary in 33.3% (n=12). In the entire cohort, week 8 outcomes of clinical response were achieved in 84.9% (28/33), steroid free clinical remission in 56.3% (18/32), normal CRP in 58.3% (7/12) and FCP response in 22.2% (2/9).
A total of 9 patients were previously exposed to tofacitinib (66.7% UC, 22.2% CD, 11.1% IBD-U; 44.4% were male). Extended induction beyond 8 weeks was necessary in 33.3% (3/9). Week 8 outcomes of clinical response were achieved in 75% (6/8), steroid free clinical remission in 50% (4/8). At week 16, 83.3% (5/6) of these patients achieved steroid free clinical remission. Table 2
Discussion: Upadacitinib resulted in steroid-free clinical remission in half (at week 8) and more than three-quarters (at week 16) of patients with IBD and previous exposure to tofacitinib. Clinical response and remission rates during induction with UPA were similar in tofacitinib exposed and naïve patients. Larger studies are needed to corroborate our findings.
Disclosures:
Hisham S. Almomen indicated no relevant financial relationships.
Ibrahim Alomar indicated no relevant financial relationships.
Chandler McMillan indicated no relevant financial relationships.
Jill Gaidos: Abbvie – Advisor or Review Panel Member, Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Pfizer – Consultant, Grant/Research Support.
Deborah Proctor: Abbvie – Consultant, Grant/Research Support. BMS – Grant/Research Support. Janssen – Grant/Research Support. Pfizer – Grant/Research Support.
Abdulelah AlMutairdi: AbbVie – Advisory Committee/Board Member. Bristol-Myers Squibb – Advisory Committee/Board Member. Pfizer – Advisory Committee/Board Member. Takeda – Advisory Committee/Board Member.
Badr Al-Bawardy: AbbVie – Speakers Bureau. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Pfizer – Advisor or Review Panel Member. Takeda – Speakers Bureau.
Hisham S. Almomen, MRCP1, Ibrahim Alomar, MBBS2, Chandler McMillan, BA1, Jill Gaidos, MD, FACG3, Deborah D. Proctor, MD4, Abdulelah AlMutairdi, MD2, Badr Al-Bawardy, MD2. P2145 - Comparison of Upadacitinib Induction in Tofacitinib Exposed and Naïve Patients With Inflammatory Bowel Disease: A Real World Multi-Center Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
