Monday Poster Session
Category: IBD
P2152 - Longitudinal Outcomes of Anti-TNF Therapy in the Treatment of Inflammatory Bowel Disease
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- AR
Andrew R. Roney, BA
UCLA
Los Angeles, California
Presenting Author(s)
Andrew R. Roney, BA, Dupre Orr, BA, Armeen Barghi, BA, Vivy Cusumano, MD, Berkeley Limketkai, MD, PhD, Jenny Sauk, MD
UCLA, Los Angeles, CA
Introduction: Tumor necrosis factor α inhibitors (TNFi) are used for the long-term management of inflammatory bowel disease (IBD). Short to medium-term complications of TNFi include increased risks of infection, malignancy, immunogenicity, and hematologic and metabolic disorders. Despite their routine use, data concerning their safety beyond five years is limited. Thus, the aim of this study is to compare adverse events (AEs) in patients in clinical remission for at least 6 months who are on TNFi maintenance therapy for less than or greater than 5 years.
Methods: This retrospective study included IBD patients on stable TNFi maintenance regimens defined as no change, escalation, or discontinuation in TNFi therapy for at least 6 months, and a normal C-reactive protein thereafter. Patients were divided into groups of less than (short-term) or greater than (long-term) 5 years on stable TNFi therapy. The primary outcome was time to any AE. Secondary outcomes analyzed AE categories (infection, malignancy, dermatologic, hematologic, metabolic, respiratory, hepatic, gastrointestinal, renal, neurologic, cardiovascular, pancreatic, musculoskeletal, endocrine, reproductive, infusion reaction, IBD related surgery). Time to an AE was examined by Kaplan-Meier failure estimates for each group from commencement of stable TNFi therapy ( > 6 months) until a change in TNFi therapy or the date of the patient’s last visit, if there was no change in therapy. Multivariable Cox proportional hazards models adjusting for patient demographics, disease characteristics, and concomitant therapies were used to determine the relative hazard ratio (HR) of an AE occurring between groups.
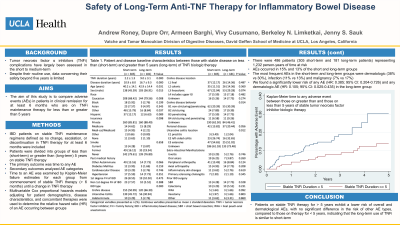
Results: There were 485 patients (304 short-term and 181 long-term patients) representing 1,232 person-years of time at risk. AEs occurred in 15% and 13% of the short and long-term groups, respectively. The most frequent AEs in the short-term and long-term groups were dermatologic (38% vs 30%), infection (11% vs 13%) and malignancy (7% vs 17%). We found a significantly lower risk of any AE (HR: 0.385; 95% CI: 0.204-0.726) and any dermatologic AE (HR: 0.105; 95% CI: 0.025-0.435) in the long-term group (Figure 1). The adjusted risk for other AEs were the same between groups.
Discussion: Patients on stable TNFi therapy for > 5 years exhibit a lower risk of overall and dermatological AEs, with no significant difference in the risk of other AE types, compared to those on therapy for < 5 years, indicating that the long-term use of TNFi appears safe.

Disclosures:
Andrew R. Roney, BA, Dupre Orr, BA, Armeen Barghi, BA, Vivy Cusumano, MD, Berkeley Limketkai, MD, PhD, Jenny Sauk, MD. P2152 - Longitudinal Outcomes of Anti-TNF Therapy in the Treatment of Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
UCLA, Los Angeles, CA
Introduction: Tumor necrosis factor α inhibitors (TNFi) are used for the long-term management of inflammatory bowel disease (IBD). Short to medium-term complications of TNFi include increased risks of infection, malignancy, immunogenicity, and hematologic and metabolic disorders. Despite their routine use, data concerning their safety beyond five years is limited. Thus, the aim of this study is to compare adverse events (AEs) in patients in clinical remission for at least 6 months who are on TNFi maintenance therapy for less than or greater than 5 years.
Methods: This retrospective study included IBD patients on stable TNFi maintenance regimens defined as no change, escalation, or discontinuation in TNFi therapy for at least 6 months, and a normal C-reactive protein thereafter. Patients were divided into groups of less than (short-term) or greater than (long-term) 5 years on stable TNFi therapy. The primary outcome was time to any AE. Secondary outcomes analyzed AE categories (infection, malignancy, dermatologic, hematologic, metabolic, respiratory, hepatic, gastrointestinal, renal, neurologic, cardiovascular, pancreatic, musculoskeletal, endocrine, reproductive, infusion reaction, IBD related surgery). Time to an AE was examined by Kaplan-Meier failure estimates for each group from commencement of stable TNFi therapy ( > 6 months) until a change in TNFi therapy or the date of the patient’s last visit, if there was no change in therapy. Multivariable Cox proportional hazards models adjusting for patient demographics, disease characteristics, and concomitant therapies were used to determine the relative hazard ratio (HR) of an AE occurring between groups.
Results: There were 485 patients (304 short-term and 181 long-term patients) representing 1,232 person-years of time at risk. AEs occurred in 15% and 13% of the short and long-term groups, respectively. The most frequent AEs in the short-term and long-term groups were dermatologic (38% vs 30%), infection (11% vs 13%) and malignancy (7% vs 17%). We found a significantly lower risk of any AE (HR: 0.385; 95% CI: 0.204-0.726) and any dermatologic AE (HR: 0.105; 95% CI: 0.025-0.435) in the long-term group (Figure 1). The adjusted risk for other AEs were the same between groups.
Discussion: Patients on stable TNFi therapy for > 5 years exhibit a lower risk of overall and dermatological AEs, with no significant difference in the risk of other AE types, compared to those on therapy for < 5 years, indicating that the long-term use of TNFi appears safe.

Figure: Figure 1. Kaplan-Meier time to any adverse event between those on greater than and those on less than 5 years of stable tumor necrosis factor inhibitor biologic therapy.
Disclosures:
Andrew Roney indicated no relevant financial relationships.
Dupre Orr indicated no relevant financial relationships.
Armeen Barghi indicated no relevant financial relationships.
Vivy Cusumano indicated no relevant financial relationships.
Berkeley Limketkai: Azora Therapeutics – Consultant, Stock-privately held company.
Jenny Sauk indicated no relevant financial relationships.
Andrew R. Roney, BA, Dupre Orr, BA, Armeen Barghi, BA, Vivy Cusumano, MD, Berkeley Limketkai, MD, PhD, Jenny Sauk, MD. P2152 - Longitudinal Outcomes of Anti-TNF Therapy in the Treatment of Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
