Monday Poster Session
Category: IBD
P2153 - Cellular and Molecular Impact of the Melanocortin Receptor Agonist PL8177 in Dextran Sulfate Sodium-Induced Colitis in Rats
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- PK
Paul S. Kayne, PhD
Palatin Technologies, Inc.
Cranbury, NJ
Presenting Author(s)
Priyanka Dhingra, PhD, Alison Obr, PhD, Carl Spana, PhD, John H. Dodd, PhD, Paul S. Kayne, PhD
Palatin Technologies, Inc., Cranbury, NJ
Introduction: The effect of PL8177, a melanocortin 1 receptor-specific agonist, on inflammation, cell population composition, and gene and protein expression was investigated in colons from a dextran sulfate sodium (DSS)–induced rat model of colitis.
Methods: Colitis was induced by 5% DSS in drinking water. Rats received vehicle control (placebo) capsules, oral PL8177 capsules (20, 50, and 100 μg per animal), or mesalazine (positive control). Colons were harvested on day 8. Samples were analyzed for cytokines, and by single nuclei RNA-seq and data-independent acquisition tandem mass spectrometry. Colitis was assessed by diarrhea and rectal bleeding, colon length shortening, colon weight gain, and histopathology. Total colitis index assessed inflammatory damage.
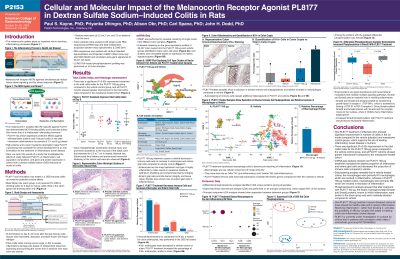
Results: PL8177 50 μg showed a significant (P< 0.05) improvement in colon weight (53% reduction), stool consistency, and fecal occult blood score vs vehicle, and there was significant improvement in the total colitis index for the PL8177 100-μg group vs vehicle. All PL8177 cohorts showed greater improvement in the total colitis index than mesalazine. Mesalazine produced a marked reduction in colon length, but only moderate improvement (35%) in colon weight. Single nuclei RNA-seq showed that in the PL8177 100-µg group, relative cell populations and key gene expression levels were closer to those of healthy controls. Subclustering analysis revealed significant differences between PL8177 100-µg and vehicle populations. Although both showed the presence of macrophages, vehicle-treated colons were primarily M1 macrophages involved in inflammation. In PL8177 100 µg–treated colons, macrophages were primarily pro-resolution M2. Proteomic analysis showed that PL8177-treated colons are more similar to sham colons than to vehicle colons. Also after treatment with PL8177, a protein known to promote intestinal homeostasis and a blood-based biomarker protein showed expression similar to that seen in the sham group, a result not seen in vehicle-treated colons.
Discussion: Oral PL8177 treatment showed significant improvement in anatomical markers of colitis vs the vehicle and mesalazine control groups, supporting the aim of treating inflammatory bowel disease (IBD). Transcriptomics and proteomics data show that oral PL8177 treatment causes diseased colons to move toward the healthy state and to resolve inflammation. Resolving inflammation—rather than blocking it—provides the possibility of efficacy coupled with safety in treating IBD.
Disclosures:
Priyanka Dhingra, PhD, Alison Obr, PhD, Carl Spana, PhD, John H. Dodd, PhD, Paul S. Kayne, PhD. P2153 - Cellular and Molecular Impact of the Melanocortin Receptor Agonist PL8177 in Dextran Sulfate Sodium-Induced Colitis in Rats, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Palatin Technologies, Inc., Cranbury, NJ
Introduction: The effect of PL8177, a melanocortin 1 receptor-specific agonist, on inflammation, cell population composition, and gene and protein expression was investigated in colons from a dextran sulfate sodium (DSS)–induced rat model of colitis.
Methods: Colitis was induced by 5% DSS in drinking water. Rats received vehicle control (placebo) capsules, oral PL8177 capsules (20, 50, and 100 μg per animal), or mesalazine (positive control). Colons were harvested on day 8. Samples were analyzed for cytokines, and by single nuclei RNA-seq and data-independent acquisition tandem mass spectrometry. Colitis was assessed by diarrhea and rectal bleeding, colon length shortening, colon weight gain, and histopathology. Total colitis index assessed inflammatory damage.
Results: PL8177 50 μg showed a significant (P< 0.05) improvement in colon weight (53% reduction), stool consistency, and fecal occult blood score vs vehicle, and there was significant improvement in the total colitis index for the PL8177 100-μg group vs vehicle. All PL8177 cohorts showed greater improvement in the total colitis index than mesalazine. Mesalazine produced a marked reduction in colon length, but only moderate improvement (35%) in colon weight. Single nuclei RNA-seq showed that in the PL8177 100-µg group, relative cell populations and key gene expression levels were closer to those of healthy controls. Subclustering analysis revealed significant differences between PL8177 100-µg and vehicle populations. Although both showed the presence of macrophages, vehicle-treated colons were primarily M1 macrophages involved in inflammation. In PL8177 100 µg–treated colons, macrophages were primarily pro-resolution M2. Proteomic analysis showed that PL8177-treated colons are more similar to sham colons than to vehicle colons. Also after treatment with PL8177, a protein known to promote intestinal homeostasis and a blood-based biomarker protein showed expression similar to that seen in the sham group, a result not seen in vehicle-treated colons.
Discussion: Oral PL8177 treatment showed significant improvement in anatomical markers of colitis vs the vehicle and mesalazine control groups, supporting the aim of treating inflammatory bowel disease (IBD). Transcriptomics and proteomics data show that oral PL8177 treatment causes diseased colons to move toward the healthy state and to resolve inflammation. Resolving inflammation—rather than blocking it—provides the possibility of efficacy coupled with safety in treating IBD.
Disclosures:
Priyanka Dhingra: Palatin Technologies, Inc. – Employee.
Alison Obr: Palatin Technologies, Inc. – Employee.
Carl Spana: Palatin Technologies, Inc. – Employee.
John Dodd: Palatin Technologies, Inc. – Employee.
Paul Kayne: Palatin Technologies, Inc. – Employee.
Priyanka Dhingra, PhD, Alison Obr, PhD, Carl Spana, PhD, John H. Dodd, PhD, Paul S. Kayne, PhD. P2153 - Cellular and Molecular Impact of the Melanocortin Receptor Agonist PL8177 in Dextran Sulfate Sodium-Induced Colitis in Rats, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
