Monday Poster Session
Category: IBD
P2156 - Clinical Outcomes of Therapeutic Drug Monitoring in Inflammatory Bowel Disease
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- ER
Emily Reznicek, DO
University of Missouri
Columbia, Missouri
Presenting Author(s)
Emily Reznicek, DO1, Ghady Moafa, MD1, Brooke Barnette, 1, Bryce Montane, MD2, Yezaz Ghouri, MD1
1University of Missouri, Columbia, MO; 2Washington University, Columbia, MO
Introduction: Therapeutic drug monitoring (TDM) has been utilized to guide drug management in patients with inflammatory bowel disease (IBD) on biologic therapy. This study compares outcomes in patients who were managed with TDM (proactive or reactive) and those who were not.
Methods: Data was collected retrospectively from our healthcare system between 2004-2022. It included adult IBD patients treated with biologic therapy. Patients were categorized into those who underwent proactive and / or reactive TDM (prTDM), proactive TDM only (pTDM), and those who were not monitored (controls). Clinical outcomes included number of patients who required hospital visits, systemic steroids, and bowel resection. Chi square and Fischer’s exact tests were used to calculate odds ratios (ORs) for each outcome. We evaluated if TDM resulted in change in biologic drug type or dose/frequency.
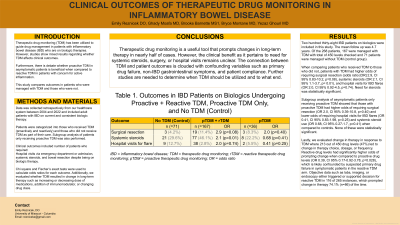
Results: The study included 238 patients of which 71 were controls and 167 underwent prTDM and 36 with pTDM. There were 450 drug levels obtained with mean follow up of 4.7 years. Patients with prTDM had higher odds of requiring surgical resection (OR 2.9, CI 95% 0.83-10.2, p=0.08), systemic steroids (OR 2.1, CI 95% 1.1-3.7, p= 0.01), and hospital visits for IBD flares (OR 2.0, CI 95% 0.92-4.5, p=0.74) when compared to controls. Those with pTDM had higher odds of requiring surgical resection (OR 2.0, CI 95% 0.39-10.8, p=0.40) and lower odds of requiring hospital visits for IBD flares (OR 0.41, CI 95% 0.83-1.98, p=0.25) and systemic steroids (OR 0.68, CI 95% 0.27-1.7, p=0.41). Out of 450 drug levels, 213 (47%) lead to change in therapy biologic drug type or dose/frequency. Reactive drug levels had higher odds of prompting medication changes when compared to proactive ones (OR 0.39, CI 95% 0.17-0.92-3.78, p=0.028), likely confounded by cases of suspected primary drug failure in symptomatic patients in rTDM arm. Labs, imaging, or endoscopy either triggered or supported decision for rTDM in 116 of 265 instances, which prompted change in therapy 74.1% (n=86) of the time.
Discussion: TDM is a useful tool that prompted changes in long-term therapy in nearly half of cases. However, the clinical benefit as it pertains to need for systemic steroids, surgery, or hospital visits remains unclear. The connection between TDM and patient outcomes is clouded with confounding variables such as primary drug failure, non-IBD gastrointestinal symptoms, and patient compliance.
Disclosures:
Emily Reznicek, DO1, Ghady Moafa, MD1, Brooke Barnette, 1, Bryce Montane, MD2, Yezaz Ghouri, MD1. P2156 - Clinical Outcomes of Therapeutic Drug Monitoring in Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Missouri, Columbia, MO; 2Washington University, Columbia, MO
Introduction: Therapeutic drug monitoring (TDM) has been utilized to guide drug management in patients with inflammatory bowel disease (IBD) on biologic therapy. This study compares outcomes in patients who were managed with TDM (proactive or reactive) and those who were not.
Methods: Data was collected retrospectively from our healthcare system between 2004-2022. It included adult IBD patients treated with biologic therapy. Patients were categorized into those who underwent proactive and / or reactive TDM (prTDM), proactive TDM only (pTDM), and those who were not monitored (controls). Clinical outcomes included number of patients who required hospital visits, systemic steroids, and bowel resection. Chi square and Fischer’s exact tests were used to calculate odds ratios (ORs) for each outcome. We evaluated if TDM resulted in change in biologic drug type or dose/frequency.
Results: The study included 238 patients of which 71 were controls and 167 underwent prTDM and 36 with pTDM. There were 450 drug levels obtained with mean follow up of 4.7 years. Patients with prTDM had higher odds of requiring surgical resection (OR 2.9, CI 95% 0.83-10.2, p=0.08), systemic steroids (OR 2.1, CI 95% 1.1-3.7, p= 0.01), and hospital visits for IBD flares (OR 2.0, CI 95% 0.92-4.5, p=0.74) when compared to controls. Those with pTDM had higher odds of requiring surgical resection (OR 2.0, CI 95% 0.39-10.8, p=0.40) and lower odds of requiring hospital visits for IBD flares (OR 0.41, CI 95% 0.83-1.98, p=0.25) and systemic steroids (OR 0.68, CI 95% 0.27-1.7, p=0.41). Out of 450 drug levels, 213 (47%) lead to change in therapy biologic drug type or dose/frequency. Reactive drug levels had higher odds of prompting medication changes when compared to proactive ones (OR 0.39, CI 95% 0.17-0.92-3.78, p=0.028), likely confounded by cases of suspected primary drug failure in symptomatic patients in rTDM arm. Labs, imaging, or endoscopy either triggered or supported decision for rTDM in 116 of 265 instances, which prompted change in therapy 74.1% (n=86) of the time.
Discussion: TDM is a useful tool that prompted changes in long-term therapy in nearly half of cases. However, the clinical benefit as it pertains to need for systemic steroids, surgery, or hospital visits remains unclear. The connection between TDM and patient outcomes is clouded with confounding variables such as primary drug failure, non-IBD gastrointestinal symptoms, and patient compliance.
Disclosures:
Emily Reznicek indicated no relevant financial relationships.
Ghady Moafa indicated no relevant financial relationships.
Brooke Barnette indicated no relevant financial relationships.
Bryce Montane: supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number TL1TR002344. – Grant/Research Support.
Yezaz Ghouri indicated no relevant financial relationships.
Emily Reznicek, DO1, Ghady Moafa, MD1, Brooke Barnette, 1, Bryce Montane, MD2, Yezaz Ghouri, MD1. P2156 - Clinical Outcomes of Therapeutic Drug Monitoring in Inflammatory Bowel Disease, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
