Monday Poster Session
Category: IBD
P2166 - Impact of Prior Biologic Exposure on the Durability of 3-Year Continuous Ozanimod Treatment
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall

Anita Afzali, MD, MPH, FACG
Executive Vice Chair of Internal Medicine, Interim Division Director of Digestive Diseases, Associate Chief Medical Officer
University of Cincinnati College of Medicine
Cincinnati, OH
Presenting Author(s)
Jean-Frederic Colombel, MD1, Anita Afzali, MD, MPH, FACG2, Douglas C. Wolf, MD, FACG3, Miguel Regueiro, MD4, Dimpy Mehra, PharmD5, Hsiuanlin Wu, MS5, Anjali Jain, PhD5, Séverine Vermeire, MD, PhD6
1Icahn School of Medicine at Mount Sinai, New York, NY; 2University of Cincinnati College of Medicine, Cincinnati, OH; 3Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 4Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH; 5Bristol Myers Squibb, Princeton, NJ; 6UZ Leuven, Leuven, Vlaams-Brabant, Belgium
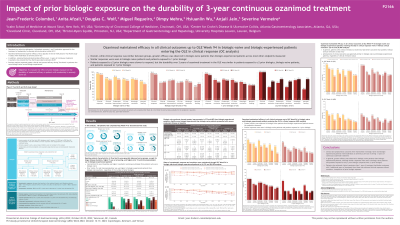
Introduction: Ozanimod (OZA) is approved for the treatment (tx) of moderately to severely active (mod/sev) ulcerative colitis (UC). OZA demonstrated superior efficacy vs placebo in the 52-wk phase 3 True North (TN) study and demonstrated durable efficacy over ~3 y of continuous tx in the open-label extension (OLE). Previous analyses reported greater clinical and mucosal efficacy during TN in biologic-naive (BN) vs biologic-experienced (BE) patients (pts).
Methods: This analysis evaluated the effect of prior biologic exposure on the durability of OZA efficacy in pts with mod/sev UC. Pts who achieved clinical response (CRes) with/without remission after 52 wk of continuous OZA tx during TN and entered the OLE were evaluated (cutoff: 10Jan2022). Symptomatic CRes and clinical remission (CRem) were evaluated through OLE W94, and CRes, CRem, endoscopic improvement, and corticosteroid (CS)–free remission were evaluated at OLE W46 and W94 using observed case (OC) and nonresponder imputation (NRI) analyses. Data were analyzed by prior biologic exposure at baseline. Pts exposed to only a Janus kinase inhibitor were not included in this analysis.
Results: 128 pts with prior biologic information entered the OLE after achieving CRes on continued OZA at TN Week (W) 52 (BN, n=82; BE, n=46). Baseline characteristics in TN were generally balanced between groups, except for longer disease duration, higher CS use at screening, and higher prior immunomodulator use in BE pts. Complete and partial Mayo scores were higher in BE pts at OLE entry. In OC analyses, symptomatic response at OLE W5 was achieved by 100% of BN and BE pts. At OLE W5, 90.8% of BN pts and 72.1% of BE pts achieved symptomatic remission. Rates of symptomatic CRes and CRem were maintained through OLE W94. In pts with CRes with or without remission at TN W52, the proportions of BN and BE pts achieving CRes, CRem, endoscopic improvement, and CS-free remission at OLE W46 and W94 are shown in the Table. Greater efficacy was observed in BN vs BE pts across all endpoints. Pts in CRem at TN W52 showed similar efficacy rates in BN and BE pts, whereas BN pts demonstrated greater efficacy than BE pts in those without CRem at TN W52. Similar patterns were seen using NRI analysis.
Discussion: Clinical and symptomatic outcomes were maintained in BN and BE pts with mod/sev active UC receiving ~3 y of continuous OZA tx. Greater efficacy was generally observed in BN pts, but BN and BE pts who achieved CRes with remission at TN W52 had similar efficacy during the OLE.
Disclosures:
Jean-Frederic Colombel, MD1, Anita Afzali, MD, MPH, FACG2, Douglas C. Wolf, MD, FACG3, Miguel Regueiro, MD4, Dimpy Mehra, PharmD5, Hsiuanlin Wu, MS5, Anjali Jain, PhD5, Séverine Vermeire, MD, PhD6. P2166 - Impact of Prior Biologic Exposure on the Durability of 3-Year Continuous Ozanimod Treatment, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2University of Cincinnati College of Medicine, Cincinnati, OH; 3Center for Crohn’s Disease & Ulcerative Colitis, Atlanta Gastroenterology Associates, Atlanta, GA; 4Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH; 5Bristol Myers Squibb, Princeton, NJ; 6UZ Leuven, Leuven, Vlaams-Brabant, Belgium
Introduction: Ozanimod (OZA) is approved for the treatment (tx) of moderately to severely active (mod/sev) ulcerative colitis (UC). OZA demonstrated superior efficacy vs placebo in the 52-wk phase 3 True North (TN) study and demonstrated durable efficacy over ~3 y of continuous tx in the open-label extension (OLE). Previous analyses reported greater clinical and mucosal efficacy during TN in biologic-naive (BN) vs biologic-experienced (BE) patients (pts).
Methods: This analysis evaluated the effect of prior biologic exposure on the durability of OZA efficacy in pts with mod/sev UC. Pts who achieved clinical response (CRes) with/without remission after 52 wk of continuous OZA tx during TN and entered the OLE were evaluated (cutoff: 10Jan2022). Symptomatic CRes and clinical remission (CRem) were evaluated through OLE W94, and CRes, CRem, endoscopic improvement, and corticosteroid (CS)–free remission were evaluated at OLE W46 and W94 using observed case (OC) and nonresponder imputation (NRI) analyses. Data were analyzed by prior biologic exposure at baseline. Pts exposed to only a Janus kinase inhibitor were not included in this analysis.
Results: 128 pts with prior biologic information entered the OLE after achieving CRes on continued OZA at TN Week (W) 52 (BN, n=82; BE, n=46). Baseline characteristics in TN were generally balanced between groups, except for longer disease duration, higher CS use at screening, and higher prior immunomodulator use in BE pts. Complete and partial Mayo scores were higher in BE pts at OLE entry. In OC analyses, symptomatic response at OLE W5 was achieved by 100% of BN and BE pts. At OLE W5, 90.8% of BN pts and 72.1% of BE pts achieved symptomatic remission. Rates of symptomatic CRes and CRem were maintained through OLE W94. In pts with CRes with or without remission at TN W52, the proportions of BN and BE pts achieving CRes, CRem, endoscopic improvement, and CS-free remission at OLE W46 and W94 are shown in the Table. Greater efficacy was observed in BN vs BE pts across all endpoints. Pts in CRem at TN W52 showed similar efficacy rates in BN and BE pts, whereas BN pts demonstrated greater efficacy than BE pts in those without CRem at TN W52. Similar patterns were seen using NRI analysis.
Discussion: Clinical and symptomatic outcomes were maintained in BN and BE pts with mod/sev active UC receiving ~3 y of continuous OZA tx. Greater efficacy was generally observed in BN pts, but BN and BE pts who achieved CRes with remission at TN W52 had similar efficacy during the OLE.
Disclosures:
Jean-Frederic Colombel: AbbVie – Consultant, Grant/Research Support, lecture fees. Allergan – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Celgene Corporation – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enterome – Consultant. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galmed Research – Consultant. Genentech (Roche) – Consultant. Gilead – Consultant. Glaxo Smith Kline – Consultant. Immunic – Consultant. Imtbio – Consultant. Inotrem – Consultant. Intestinal Biotech Development – Stock Options. Invea – Consultant. Ipsen – Consultant. Iterative Scopes – Consultant. Janssen Pharmaceuticals – Consultant, Grant/Research Support. Kaleido Biosciences – Consultant. Landos – Consultant. LimmaTech Biologics AG – Consultant. Medimmune – Consultant. Merck – Consultant. Microba Life Science – Consultant. Novartis – Consultant. O Mass – Consultant. Otsuka Pharmaceutical – Consultant. Pfizer – Consultant. Prometheus – Consultant. Protagonis Therapeutics – Consultant. Sanofi – Consultant. Seres – Consultant. Shire – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, lecture fees. Teva – Consultant. TiGenix – Consultant. Viela Bio – Consultant. Vifor – Consultant.
Anita Afzali: AbbVie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Bristol Myers Squibb/Celgene – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. DiaSorin – Consultant. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Gilead – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. IBD Horizons – Owner/Ownership Interest. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. TLL Pharmaceuticals – Consultant.
Douglas C. Wolf: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. AstraZeneca – Grant/Research Support. Bristol Myers Squibb – Consultant, Grant/Research Support, Speakers Bureau. Eli Lilly – Consultant. Genentech – Grant/Research Support. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Consultant. Pfizer/Arena – Grant/Research Support. Takeda – Consultant, Grant/Research Support, Speakers Bureau.
Miguel Regueiro: AbbVie – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Alfasigma – Advisory Committee/Board Member, Consultant. Allergan – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Celgene – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Eli Lilly – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Gilead Sciences – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Janssen – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Miraca Labs – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Prometheus – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Seres – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Target RWE – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant, Unrestricted educational grants. Wolters Kluwer Health – Royalties.
Dimpy Mehra: Bristol Myers Squibb – employee and/or shareholder.
Hsiuanlin Wu: Bristol Myers Squibb – employee and/or shareholder.
Anjali Jain: Bristol Myers Squibb – employee and/or shareholder.
Séverine Vermeire: AbbVie – Consultant, Grant/Research Support. AbolerIS Pharma – Grant/Research Support. AgomAb – Grant/Research Support. Alimentiv – Consultant. Arena Pharmaceuticals – Consultant. AstraZeneca – Consultant. Avaxia Biologics – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Celgene – Consultant. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech/Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIDomics – Consultant, Speakers Bureau. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. ProDigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillotts Pharma – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Jean-Frederic Colombel, MD1, Anita Afzali, MD, MPH, FACG2, Douglas C. Wolf, MD, FACG3, Miguel Regueiro, MD4, Dimpy Mehra, PharmD5, Hsiuanlin Wu, MS5, Anjali Jain, PhD5, Séverine Vermeire, MD, PhD6. P2166 - Impact of Prior Biologic Exposure on the Durability of 3-Year Continuous Ozanimod Treatment, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
