Monday Poster Session
Category: IBD
P2185 - A Comparison of Patient Characteristics and Outcomes After Biologic Therapy Induction in Obese vs Non-Obese Patients With Inflammatory Bowel Disease (IBD)
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio
- CP
Colton J. Pence, BS, MD
New York-Presbyterian/Weill Cornell Medical Center
New York, NY
Presenting Author(s)
Colton J. Pence, BS, MD1, Catherine Ng, MS2, Debra D'Angelo, MS2, Dana Lukin, MD, PhD, FACG3
1New York-Presbyterian/Weill Cornell Medical Center, New York, NY; 2Weill Cornell Medical Center, New York, NY; 3Jill Roberts Center for Inflammatory Bowel Disease, New York, NY
Introduction: Obesity is an increasingly common condition in the general population and is one of the most common risk factors linked to a wide variety of diseases. Various studies have examined the relation of obesity on patients with IBD, but results have been inconsistent. Current studies have not demonstrated differences in response to TNF-antagonist therapies between obese and non-obese patients, but data are limited with regard to non-TNF biologics. This retrospective study examined outcomes in obese vs non-obese individuals during biologic induction.
Methods: A retrospective study was conducted at an academic, urban tertiary center. Patients diagnosed with IBD, who had at least two office visits and one year of follow-up after initiation of biologic therapy, were included. Patients were categorized into obese and non-obese using a BMI of 30. Data were collected via abstraction from the electronic health record and information collected in a data management system. Outcomes, including hospitalization, surgery, new steroid prescriptions, transition to another biologic, and death, were monitored within a year post-induction; these outcomes were compared as a composite outcome of treatment failure. Data were analyzed using Wilcoxon Rank Sum Test and Fisher’s Exact Test.
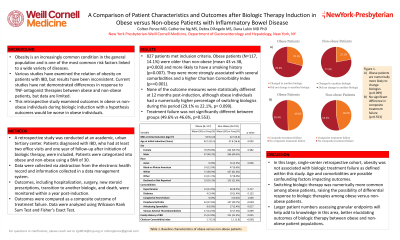
Results: 827 patients met inclusion criteria. Obese patients (N=117, 14.1%) were older than non-obese (mean 43 vs 38, p=0.003) and more likely to have a smoking history (p=0.007). They were more strongly associated with several comorbidities and a higher Charlson Comorbidity Index (p-value < 0.001). C-reactive protein and stool calprotectin did not vary significantly. None of the outcome measures were statistically different at 12 months post-induction, although obese individuals had a numerically higher percentage of switching biologics during this period (29.1% vs 22.1%, p= 0.099). Treatment failure was not significantly different between groups (49.6% vs 46.6%, p=0.553).
Discussion: In this large, single-center retrospective cohort, obesity was not associated with biologic treatment failure. Switching biologic therapy was numerically more common among obese patients. Age and comorbidities are possible confounding factors impacting outcomes. Larger patient numbers assessing granular endpoints will help add to knowledge in this area.
Disclosures:
Colton J. Pence, BS, MD1, Catherine Ng, MS2, Debra D'Angelo, MS2, Dana Lukin, MD, PhD, FACG3. P2185 - A Comparison of Patient Characteristics and Outcomes After Biologic Therapy Induction in Obese vs Non-Obese Patients With Inflammatory Bowel Disease (IBD), ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1New York-Presbyterian/Weill Cornell Medical Center, New York, NY; 2Weill Cornell Medical Center, New York, NY; 3Jill Roberts Center for Inflammatory Bowel Disease, New York, NY
Introduction: Obesity is an increasingly common condition in the general population and is one of the most common risk factors linked to a wide variety of diseases. Various studies have examined the relation of obesity on patients with IBD, but results have been inconsistent. Current studies have not demonstrated differences in response to TNF-antagonist therapies between obese and non-obese patients, but data are limited with regard to non-TNF biologics. This retrospective study examined outcomes in obese vs non-obese individuals during biologic induction.
Methods: A retrospective study was conducted at an academic, urban tertiary center. Patients diagnosed with IBD, who had at least two office visits and one year of follow-up after initiation of biologic therapy, were included. Patients were categorized into obese and non-obese using a BMI of 30. Data were collected via abstraction from the electronic health record and information collected in a data management system. Outcomes, including hospitalization, surgery, new steroid prescriptions, transition to another biologic, and death, were monitored within a year post-induction; these outcomes were compared as a composite outcome of treatment failure. Data were analyzed using Wilcoxon Rank Sum Test and Fisher’s Exact Test.
Results: 827 patients met inclusion criteria. Obese patients (N=117, 14.1%) were older than non-obese (mean 43 vs 38, p=0.003) and more likely to have a smoking history (p=0.007). They were more strongly associated with several comorbidities and a higher Charlson Comorbidity Index (p-value < 0.001). C-reactive protein and stool calprotectin did not vary significantly. None of the outcome measures were statistically different at 12 months post-induction, although obese individuals had a numerically higher percentage of switching biologics during this period (29.1% vs 22.1%, p= 0.099). Treatment failure was not significantly different between groups (49.6% vs 46.6%, p=0.553).
Discussion: In this large, single-center retrospective cohort, obesity was not associated with biologic treatment failure. Switching biologic therapy was numerically more common among obese patients. Age and comorbidities are possible confounding factors impacting outcomes. Larger patient numbers assessing granular endpoints will help add to knowledge in this area.
Disclosures:
Colton Pence indicated no relevant financial relationships.
Catherine Ng indicated no relevant financial relationships.
Debra D'Angelo indicated no relevant financial relationships.
Dana Lukin: Abbvie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Consultant. Fresenius Kabi – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Magellan Health – Consultant. Palatin – Consultant. Pfizer – Consultant. Prometheus – Consultant. PSI – Consultant. Takeda – Consultant, Grant/Research Support.
Colton J. Pence, BS, MD1, Catherine Ng, MS2, Debra D'Angelo, MS2, Dana Lukin, MD, PhD, FACG3. P2185 - A Comparison of Patient Characteristics and Outcomes After Biologic Therapy Induction in Obese vs Non-Obese Patients With Inflammatory Bowel Disease (IBD), ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
