Monday Poster Session
Category: IBD
P2193 - Ozanimod-Exposed Patients With Ulcerative Colitis Undergoing Total Colectomy Exhibit Unique Histologic Changes in Lymph Nodes
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Nathaniel A. Cohen, MD
University of Chicago Medicine, Inflammatory Bowel Disease Center
TEL AVIV, Israel
Presenting Author(s)
Nathaniel A. Cohen, MD1, Christopher R. Weber, MD, PhD2, Jason X.. Cheng, MD, PhD2, David K.. Choi, PharmD1, Nicole M. Garcia, BA1, Natalie K. Choi, BA1, David T. Rubin, MD3
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago Medicine, Chicago, IL; 3Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL
Introduction: Ozanimod regulates lymphocyte egress from the spleen and lymph nodes into the systemic circulation. The histologic changes which occur in the lymph nodes of patients on these therapies is unknown. We describe histologic changes in lymph nodes from patients with UC who were treated with ozanimod and underwent subsequent colectomy due to treatment-refractory disease.
Methods: This retrospective study included patients with UC undergoing total colectomy for treatment-refractory disease who were treated with ozanimod within the 2 months prior to colectomy and a cohort of patients with UC undergoing colectomy who did not have ozanimod exposure. Histology of the lymph nodes from the mesentery of colectomy specimens were reviewed blindly by a hematophatologist (JXC) and an expert GI pathologist (CRW). Histopathological parameters were scored and 2-tailed student’s unpaired t-test was used to determine significance between groups. Demographic and clinical data were recorded.
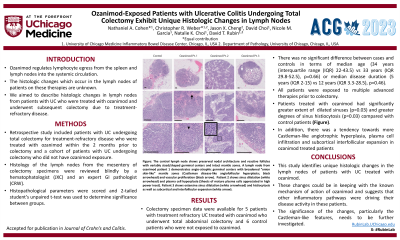
Results: From our series of 45 patients treated with ozanimod, 6 (13%) required surgery for treatment-refractory disease. Colectomy specimen data were available for 5 patients (1 patient had surgery at an outside center). Lymph node specimens from 6 control patients with UC who had colectomy were also examined. Demographic and clinical data are summarized in Table 1. The duration of ozanimod therapy prior to surgery ranged from 6 to 24 weeks. Histologic examination of lymph nodes showed that patients treated with ozanimod had significantly greater extent of dilated sinuses (p=0.03) and greater degrees of sinus histiocytosis (p=0.03) compared with control patients. In addition, there was a tendency towards more Castleman-like angiotrophic hyperplasia, plasma cell infiltration and subcortical interfollicular expansion in ozanimod treated patients (not statistically significant). (Figure 1).
Discussion: This study identifies unique histologic changes in the lymph nodes of patients with UC treated with ozanimod. These changes could be in keeping with the known mechanism of action of ozanimod and suggests that other inflammatory pathways were driving their disease activity in these patients. The significance of the changes, particularly the Castleman-like features, needs to be further investigated.

Disclosures:
Nathaniel A. Cohen, MD1, Christopher R. Weber, MD, PhD2, Jason X.. Cheng, MD, PhD2, David K.. Choi, PharmD1, Nicole M. Garcia, BA1, Natalie K. Choi, BA1, David T. Rubin, MD3. P2193 - Ozanimod-Exposed Patients With Ulcerative Colitis Undergoing Total Colectomy Exhibit Unique Histologic Changes in Lymph Nodes, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago Medicine, Chicago, IL; 3Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL
Introduction: Ozanimod regulates lymphocyte egress from the spleen and lymph nodes into the systemic circulation. The histologic changes which occur in the lymph nodes of patients on these therapies is unknown. We describe histologic changes in lymph nodes from patients with UC who were treated with ozanimod and underwent subsequent colectomy due to treatment-refractory disease.
Methods: This retrospective study included patients with UC undergoing total colectomy for treatment-refractory disease who were treated with ozanimod within the 2 months prior to colectomy and a cohort of patients with UC undergoing colectomy who did not have ozanimod exposure. Histology of the lymph nodes from the mesentery of colectomy specimens were reviewed blindly by a hematophatologist (JXC) and an expert GI pathologist (CRW). Histopathological parameters were scored and 2-tailed student’s unpaired t-test was used to determine significance between groups. Demographic and clinical data were recorded.
Results: From our series of 45 patients treated with ozanimod, 6 (13%) required surgery for treatment-refractory disease. Colectomy specimen data were available for 5 patients (1 patient had surgery at an outside center). Lymph node specimens from 6 control patients with UC who had colectomy were also examined. Demographic and clinical data are summarized in Table 1. The duration of ozanimod therapy prior to surgery ranged from 6 to 24 weeks. Histologic examination of lymph nodes showed that patients treated with ozanimod had significantly greater extent of dilated sinuses (p=0.03) and greater degrees of sinus histiocytosis (p=0.03) compared with control patients. In addition, there was a tendency towards more Castleman-like angiotrophic hyperplasia, plasma cell infiltration and subcortical interfollicular expansion in ozanimod treated patients (not statistically significant). (Figure 1).
Discussion: This study identifies unique histologic changes in the lymph nodes of patients with UC treated with ozanimod. These changes could be in keeping with the known mechanism of action of ozanimod and suggests that other inflammatory pathways were driving their disease activity in these patients. The significance of the changes, particularly the Castleman-like features, needs to be further investigated.

Figure: Figure 1: Images of lymph nodes from patients with UC, treated with ozanimod compared to a control patient
Disclosures:
Nathaniel Cohen: Iterative Health – Consultant. Pfizer – Travel support. Seres Pharmaceuticals – Consultant. Takeda – Consultant.
Christopher Weber indicated no relevant financial relationships.
Jason Cheng indicated no relevant financial relationships.
David Choi: Abbvie – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Janssen Pharmaceuticals – Consultant, Speakers Bureau. Prometheus Laboratory – Consultant.
Nicole Garcia indicated no relevant financial relationships.
Natalie Choi indicated no relevant financial relationships.
David Rubin: AbbVie – Consultant, personal fees. AltruBio – Consultant, personal fees. Aslan Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant, personal fees. Bristol Myers Squibb – Consultant. Celgene Chronicles – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Corp/Syneos – Consultant. Eco R1 – Consultant. GastroIntestinal Research Foundation – Grant/Research Support. Genentech/Roche – Consultant. Gilead Sciences – Consultant, personal fees. Helmsley Charitable Trust – Grant/Research Support. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant, personal fees. Kaleido Biosciences – Consultant. Lilly – Consultant. Pfizer – Consultant, personal fees. Prometheus Biosciences – Consultant. Reistone Biopharma – Consultant, personal fees. Seres Therapeutics – Consultant. Takeda – Consultant, Grant/Research Support, Personal fees. Target RWE – Consultant. Trellus Health – Consultant.
Nathaniel A. Cohen, MD1, Christopher R. Weber, MD, PhD2, Jason X.. Cheng, MD, PhD2, David K.. Choi, PharmD1, Nicole M. Garcia, BA1, Natalie K. Choi, BA1, David T. Rubin, MD3. P2193 - Ozanimod-Exposed Patients With Ulcerative Colitis Undergoing Total Colectomy Exhibit Unique Histologic Changes in Lymph Nodes, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

