Monday Poster Session
Category: IBD
P2200 - Efficacy and Safety of Etrasimod in Patients With and Without Concomitant Corticosteroid Treatment in the Phase 3 ELEVATE UC 52 and ELEVATE UC 12 Trials
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Bruce E. Sands, MD, MS, FACG
Icahn School of Medicine at Mount Sinai
New York, New York
Presenting Author(s)
Award: Presidential Poster Award
Bruce E. Sands, MD, MS, FACG1, Krisztina B. Gecse, MD, PhD2, David T. Rubin, MD3, Yvette Leung, MD, FRCPC4, Julian Panés, MD, PhD5, Martina Goetsch, MD6, Wenjin Wang, PhD7, Kevin Shan, PhD8, John C.. Woolcott, PhD7, Christina C.. Smith, PharmD, MBA7, Karolina Wosik, MSc, PhD9, Stefan Schreiber, MD10
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 3Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL; 4University of British Columbia, Vancouver, BC, Canada; 5Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 6Pfizer AG, Zurich, Zurich, Switzerland; 7Pfizer Inc., Collegeville, PA; 8Pfizer Inc., New York, NY; 9Pfizer Canada, Kirkland, PQ, Canada; 10University Hospital Schleswig-Holstein, Kiel, Schleswig-Holstein, Germany
Introduction: Etrasimod is an investigational, oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator in development for the treatment of moderately to severely active ulcerative colitis (UC).
Methods: We report efficacy and safety of etrasimod in patients (pts) with/without concomitant corticosteroid (CS) use at baseline (BL) of the ELEVATE UC phase 3 trials. In ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369), pts with moderately to severely active UC were randomized 2:1 to once-daily etrasimod 2 mg or placebo (PBO). ELEVATE UC 52 used a treat-through design with a 12-week (wk) induction period followed by a 40-wk maintenance period. ELEVATE UC 12 had a 12-wk induction period. At entry, pts were permitted to receive oral concomitant CS (prednisone [≤ 20 mg/day], budesonide [≤ 9 mg/day], or equivalent]) if on a stable dose for ≥ 4 wks prior to screening endoscopy; from Wk 12 CS tapering was recommended. Primary and secondary efficacy endpoints (defined in Table) and safety were assessed by BL concomitant CS status.
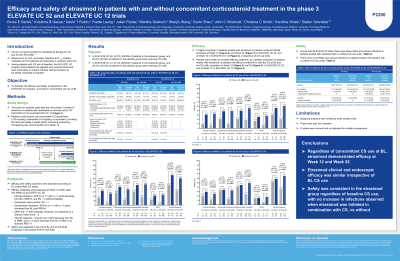
Results: In ELEVATE UC 52, 32.2% (93/289) and 29.2% (42/144) of etrasimod- and PBO-treated pts, respectively, were receiving CS at BL. In ELEVATE UC 12, 27.3% (65/238) and 29.3% (34/116) of etrasimod- and PBO-treated pts were receiving CS at BL. Among pts receiving CS at BL in ELEVATE UC 52, a higher proportion of etrasimod- vs PBO-treated pts achieved the endpoint of clinical remission (CR) at Wk 12 (p < 0.05) and Wk 52 (p < 0.001); this was also observed in pts not receiving CS at BL (p < 0.001 at Wks 12 and 52; Table). In ELEVATE UC 12, a higher observed proportion of etrasimod- vs PBO-treated pts receiving CS at BL achieved CR at Wk 12 (p > 0.05); a significant difference was observed in pts not receiving CS at BL (p < 0.01). In both studies, similar results were observed across all secondary endpoints, with greater differences between etrasimod and PBO seen in pts not receiving CS at BL (Table). Across both ELEVATE trials, there were fewer serious adverse events and serious infections in the etrasimod arm in pts with CS at BL, than in those without CS at BL. In the PBO arms, the opposite was true.
Discussion: Regardless of concomitant CS use at BL, etrasimod generally demonstrated efficacy at Wks 12 and 52, with greater treatment effect seen in pts without CS use at BL. No additional safety signal was apparent when etrasimod was initiated in combination with CS compared to without CS.
Disclosures:
Bruce E. Sands, MD, MS, FACG1, Krisztina B. Gecse, MD, PhD2, David T. Rubin, MD3, Yvette Leung, MD, FRCPC4, Julian Panés, MD, PhD5, Martina Goetsch, MD6, Wenjin Wang, PhD7, Kevin Shan, PhD8, John C.. Woolcott, PhD7, Christina C.. Smith, PharmD, MBA7, Karolina Wosik, MSc, PhD9, Stefan Schreiber, MD10. P2200 - Efficacy and Safety of Etrasimod in Patients With and Without Concomitant Corticosteroid Treatment in the Phase 3 ELEVATE UC 52 and ELEVATE UC 12 Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
Bruce E. Sands, MD, MS, FACG1, Krisztina B. Gecse, MD, PhD2, David T. Rubin, MD3, Yvette Leung, MD, FRCPC4, Julian Panés, MD, PhD5, Martina Goetsch, MD6, Wenjin Wang, PhD7, Kevin Shan, PhD8, John C.. Woolcott, PhD7, Christina C.. Smith, PharmD, MBA7, Karolina Wosik, MSc, PhD9, Stefan Schreiber, MD10
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 3Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL; 4University of British Columbia, Vancouver, BC, Canada; 5Hospital Clínic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Catalonia, Spain; 6Pfizer AG, Zurich, Zurich, Switzerland; 7Pfizer Inc., Collegeville, PA; 8Pfizer Inc., New York, NY; 9Pfizer Canada, Kirkland, PQ, Canada; 10University Hospital Schleswig-Holstein, Kiel, Schleswig-Holstein, Germany
Introduction: Etrasimod is an investigational, oral, once-daily, selective sphingosine 1-phosphate (S1P)1,4,5 receptor modulator in development for the treatment of moderately to severely active ulcerative colitis (UC).
Methods: We report efficacy and safety of etrasimod in patients (pts) with/without concomitant corticosteroid (CS) use at baseline (BL) of the ELEVATE UC phase 3 trials. In ELEVATE UC 52 (NCT03945188) and ELEVATE UC 12 (NCT03996369), pts with moderately to severely active UC were randomized 2:1 to once-daily etrasimod 2 mg or placebo (PBO). ELEVATE UC 52 used a treat-through design with a 12-week (wk) induction period followed by a 40-wk maintenance period. ELEVATE UC 12 had a 12-wk induction period. At entry, pts were permitted to receive oral concomitant CS (prednisone [≤ 20 mg/day], budesonide [≤ 9 mg/day], or equivalent]) if on a stable dose for ≥ 4 wks prior to screening endoscopy; from Wk 12 CS tapering was recommended. Primary and secondary efficacy endpoints (defined in Table) and safety were assessed by BL concomitant CS status.
Results: In ELEVATE UC 52, 32.2% (93/289) and 29.2% (42/144) of etrasimod- and PBO-treated pts, respectively, were receiving CS at BL. In ELEVATE UC 12, 27.3% (65/238) and 29.3% (34/116) of etrasimod- and PBO-treated pts were receiving CS at BL. Among pts receiving CS at BL in ELEVATE UC 52, a higher proportion of etrasimod- vs PBO-treated pts achieved the endpoint of clinical remission (CR) at Wk 12 (p < 0.05) and Wk 52 (p < 0.001); this was also observed in pts not receiving CS at BL (p < 0.001 at Wks 12 and 52; Table). In ELEVATE UC 12, a higher observed proportion of etrasimod- vs PBO-treated pts receiving CS at BL achieved CR at Wk 12 (p > 0.05); a significant difference was observed in pts not receiving CS at BL (p < 0.01). In both studies, similar results were observed across all secondary endpoints, with greater differences between etrasimod and PBO seen in pts not receiving CS at BL (Table). Across both ELEVATE trials, there were fewer serious adverse events and serious infections in the etrasimod arm in pts with CS at BL, than in those without CS at BL. In the PBO arms, the opposite was true.
Discussion: Regardless of concomitant CS use at BL, etrasimod generally demonstrated efficacy at Wks 12 and 52, with greater treatment effect seen in pts without CS use at BL. No additional safety signal was apparent when etrasimod was initiated in combination with CS compared to without CS.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speaker’s fees. Adiso Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena pharmaceuticals – Consultant. Artizan Biosciences – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees and other support. Calibr – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Connect Biopharm – Consultant. Cytoki Pharma – Consultant. Eli Lilly – Consultant, speaking fees and other support. Enthera – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. HMP Acquisition – Consultant. Imhotex – Consultant. Immunic – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Grant/Research Support, consulting and speaking fees and other support. Johnson & Johnson – Consultant. Kaleido Biosciences – Consultant. Kallyope – Consultant. Merck – Consultant. MiroBio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. OSE Immunotherapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, speaking fees and other support. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. RedHill Biopharma – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Synlogic Operating Company – Consultant. Takeda – Grant/Research Support, consulting and speaking fees and other support. Target RWE – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. Ventyx Biosciences – Consultant, personal fees and stock options for consulting. Viela Bio – Consultant.
Krisztina Gecse: Abbvie – Consultant, Speakers Bureau. Celltrion – Grant/Research Support, Speakers Bureau. Ferring Pharmaceuticals – Speakers Bureau. Galapagos – Grant/Research Support. Immunic Therapeutics – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Novartis – Consultant, Speakers Bureau. Pfizer Inc – Grant/Research Support. Samsung Bioepis – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Tillotts – Speakers Bureau.
David Rubin: AbbVie – Consultant, personal fees. AltruBio – Consultant, personal fees. Aslan Pharmaceuticals – Consultant. Athos Therapeutics – Consultant. Bellatrix Pharmaceuticals – Consultant. Boehringer Ingelheim – Consultant, personal fees. Bristol Myers Squibb – Consultant. Celgene Chronicles – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Corp/Syneos – Consultant. Eco R1 – Consultant. GastroIntestinal Research Foundation – Grant/Research Support. Genentech/Roche – Consultant. Gilead Sciences – Consultant, personal fees. Helmsley Charitable Trust – Grant/Research Support. Iterative Health – Consultant. Janssen Pharmaceuticals – Consultant, personal fees. Kaleido Biosciences – Consultant. Lilly – Consultant. Pfizer – Consultant, personal fees. Prometheus Biosciences – Consultant. Reistone Biopharma – Consultant, personal fees. Seres Therapeutics – Consultant. Takeda – Consultant, Grant/Research Support, Personal fees. Target RWE – Consultant. Trellus Health – Consultant.
Yvette Leung indicated no relevant financial relationships.
Julian Panés: AbbVie – Grant/Research Support, Personal fees. Arena – Consultant. Athos – Consultant. Atomwise – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant. Genentech/Roche – Consultant. GlaxoSmithKline – Consultant. Immunic – Personal fees. Janssen – Consultant, payment for development of educational presentations. Mirum – Consultant. Morphic – Consultant. Origo – Consultant. Pandion – Consultant. Pfizer Inc – Grant/Research Support, payment for development of educational presentations. Progenity – Consultant. Revolo – Consultant. Takeda – payment for development of educational presentations. Theravance – Consultant. Wasserman – Consultant.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock-publicly held company(excluding mutual/index funds).
Wenjin Wang: Pfizer Inc – Employee, Stock Options.
Kevin Shan: Pfizer Inc – Employee, Stock-publicly held company(excluding mutual/index funds).
John Woolcott: Pfizer Inc – Employee, Stock Options.
Christina Smith: Pfizer Inc – Employee, Stock-publicly held company(excluding mutual/index funds).
Karolina Wosik: Pfizer Canada – Employee, Stock-publicly held company(excluding mutual/index funds).
Stefan Schreiber: AbbVie – Personal fees. Amgen – Personal fees. Arena Pharmaceuticals – Personal fees. Biogen – Personal fees. Bristol Myers Squibb – Personal fees. Celgene – Personal fees. Celltrion Healthcare – Personal fees. Dr. Falk Pharma – Personal fees. Eli Lilly – personal fees. Ferring Pharmaceuticals – personal fees. Fresenius Kabi – Personal fees. Galapagos – Personal fees. Gilead – Personal fees. Hikma Pharmaceuticals – Personal fees. I-Mab – Personal fees. Janssen Pharmaceuticals – Personal fees. Morphic – Personal fees. MSD – Personal fees. Mylan – Personal fees. Pfizer – Personal fees. Protagonist – Personal fees. ProventionBio – Personal fees. Sandoz/Hexal – personal fees. Takeda – Personal fees. Theravance Biopharma – Personal fees. UCB – personal fees.
Bruce E. Sands, MD, MS, FACG1, Krisztina B. Gecse, MD, PhD2, David T. Rubin, MD3, Yvette Leung, MD, FRCPC4, Julian Panés, MD, PhD5, Martina Goetsch, MD6, Wenjin Wang, PhD7, Kevin Shan, PhD8, John C.. Woolcott, PhD7, Christina C.. Smith, PharmD, MBA7, Karolina Wosik, MSc, PhD9, Stefan Schreiber, MD10. P2200 - Efficacy and Safety of Etrasimod in Patients With and Without Concomitant Corticosteroid Treatment in the Phase 3 ELEVATE UC 52 and ELEVATE UC 12 Trials, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.

