Monday Poster Session
Category: IBD
P2202 - Improvement in Inflammatory Biomarker Levels Through Week 12 in Moderately to Severely Active Ulcerative Colitis Patients Treated With Guselkumab: Results From the Phase 3 QUASAR Induction Study
Monday, October 23, 2023
10:30 AM - 4:15 PM PT
Location: Exhibit Hall
Has Audio

Bruce E. Sands, MD, MS, FACG
Icahn School of Medicine at Mount Sinai
New York, New York
Presenting Author(s)
Tadakazu Hisamatsu, MD, PhD1, Laurent Peyrin-Biroulet, MD, PhD2, Axel Dignass, MD, PhD3, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Nicole Houck, BS4, Ye Miao, MS4, Hongyan Zhang, PhD4, Marjorie Argollo, MD5, Ken Takeuchi, MD, PhD6, Rafal Filip, MD, PhD7, Jessica R.. Allegretti, MD, MPH8, Brian G. Feagan, MD9, Bruce E. Sands, MD, MS, FACG10
1Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 2Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France; 3Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 4Janssen Research & Development, LLC, Spring House, PA; 5Onco Star SP Oncologia Ltda, Sao Paulo, Sao Paulo, Brazil; 6Tsujinaka Hospital Kashiwanoha, Kashiwa, Chiba, Japan; 7Clinical Hospital No. 2, Rzeszow, Podkarpackie, Poland; 8Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 9Western University, London, ON, Canada; 10Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: C-reactive protein (CRP) and fecal calprotectin (FeCal) are non-invasive inflammatory biomarkers used to assess disease activity in UC. The Phase 3 QUASAR induction study evaluated efficacy and safety of guselkumab (GUS), an IL-23 p19 subunit antagonist, in patients (pts) with moderately to severely active UC. Here, we analyze the effect of GUS treatment on CRP and FeCal through Week (Wk) 12 in pts with elevated CRP and/or FeCal at baseline (BL).
Methods: Pts with a modified Mayo score of 5-9 and a Mayo endoscopy subscore ≥2 at BL were randomized 3:2 to receive either GUS 200mg IV or placebo (PBO) IV at Wks0, 4, and 8. CRP and FeCal were assessed at BL, Wk4, Wk8 (for CRP only), and Wk12. All analyses were not multiplicity controlled (nominal p-values).
Results: Of 701 pts assessed, nearly 50% had a history of inadequate response/intolerance to advanced therapies (ADT) for UC; 47.4% of these pts had inadequate response/intolerance to ≥2 ADT classes. Median BL concentrations of CRP and FeCal were similar between the GUS- and PBO-treated pts (4.34 vs 3.83mg/L and 1651 vs 1606mg/kg, respectively). The proportions of pts with elevated CRP/FeCal at BL were similar between treatment groups.
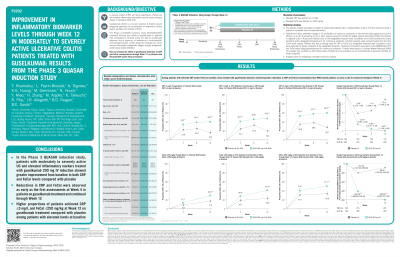
Compared with PBO, greater reductions in CRP and FeCal were observed with GUS at the earliest timepoint assessed (Wk4) and continued through Wk12 (Table). Among pts with elevated CRP ( >3mg/L), median change from BL in CRP concentration (mg/L) at Wk12 for the GUS and PBO cohorts were -3.99 and -0.51mg/L, respectively (p< 0.001); the proportions of pts achieving 50% and 75% reduction in CRP levels (or ≤3mg/L) at Wk12 were higher for GUS-treated pts than those receiving PBO (59.7% vs 28.1% and 47.2% vs 19.4%; both p< 0.001). Among pts with elevated FeCal ( >250mg/kg), median change from BL in FeCal concentration (mg/kg) at Wk12 were -800 and -86mg/kg for the GUS and PBO cohorts, respectively (p< 0.001); the proportions of pts achieving 50% and 75% reduction in FeCal levels (or ≤250 mg/kg) at Wk12 were higher for GUS-treated pts than those receiving PBO (51.1% vs 33.8% and 41.4% vs 23.6%; both p< 0.001). At Wk12, GUS-treated pts achieved CRP≤3mg/L and/or FeCal≤250mg/kg at higher rates than PBO-treated pts (Figure).
Discussion: Pts with moderately to severely active UC and elevated inflammatory markers treated with GUS 200mg IV induction showed greater improvement from BL in both CRP and FeCal levels compared with PBO. Differences were observed as early as the first assessments at Wk4 and continued through Wk12.

Disclosures:
Tadakazu Hisamatsu, MD, PhD1, Laurent Peyrin-Biroulet, MD, PhD2, Axel Dignass, MD, PhD3, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Nicole Houck, BS4, Ye Miao, MS4, Hongyan Zhang, PhD4, Marjorie Argollo, MD5, Ken Takeuchi, MD, PhD6, Rafal Filip, MD, PhD7, Jessica R.. Allegretti, MD, MPH8, Brian G. Feagan, MD9, Bruce E. Sands, MD, MS, FACG10. P2202 - Improvement in Inflammatory Biomarker Levels Through Week 12 in Moderately to Severely Active Ulcerative Colitis Patients Treated With Guselkumab: Results From the Phase 3 QUASAR Induction Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
1Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 2Last Inserm U954 and CHU de Nancy, Lorraine University, Vandoeuvre-lès-Nancy, Lorraine, France; 3Agaplesion Markus Hospital, Goethe University, Frankfurt, Hessen, Germany; 4Janssen Research & Development, LLC, Spring House, PA; 5Onco Star SP Oncologia Ltda, Sao Paulo, Sao Paulo, Brazil; 6Tsujinaka Hospital Kashiwanoha, Kashiwa, Chiba, Japan; 7Clinical Hospital No. 2, Rzeszow, Podkarpackie, Poland; 8Brigham and Women's Hospital, Harvard Medical School, Boston, MA; 9Western University, London, ON, Canada; 10Icahn School of Medicine at Mount Sinai, New York, NY
Introduction: C-reactive protein (CRP) and fecal calprotectin (FeCal) are non-invasive inflammatory biomarkers used to assess disease activity in UC. The Phase 3 QUASAR induction study evaluated efficacy and safety of guselkumab (GUS), an IL-23 p19 subunit antagonist, in patients (pts) with moderately to severely active UC. Here, we analyze the effect of GUS treatment on CRP and FeCal through Week (Wk) 12 in pts with elevated CRP and/or FeCal at baseline (BL).
Methods: Pts with a modified Mayo score of 5-9 and a Mayo endoscopy subscore ≥2 at BL were randomized 3:2 to receive either GUS 200mg IV or placebo (PBO) IV at Wks0, 4, and 8. CRP and FeCal were assessed at BL, Wk4, Wk8 (for CRP only), and Wk12. All analyses were not multiplicity controlled (nominal p-values).
Results: Of 701 pts assessed, nearly 50% had a history of inadequate response/intolerance to advanced therapies (ADT) for UC; 47.4% of these pts had inadequate response/intolerance to ≥2 ADT classes. Median BL concentrations of CRP and FeCal were similar between the GUS- and PBO-treated pts (4.34 vs 3.83mg/L and 1651 vs 1606mg/kg, respectively). The proportions of pts with elevated CRP/FeCal at BL were similar between treatment groups.
Compared with PBO, greater reductions in CRP and FeCal were observed with GUS at the earliest timepoint assessed (Wk4) and continued through Wk12 (Table). Among pts with elevated CRP ( >3mg/L), median change from BL in CRP concentration (mg/L) at Wk12 for the GUS and PBO cohorts were -3.99 and -0.51mg/L, respectively (p< 0.001); the proportions of pts achieving 50% and 75% reduction in CRP levels (or ≤3mg/L) at Wk12 were higher for GUS-treated pts than those receiving PBO (59.7% vs 28.1% and 47.2% vs 19.4%; both p< 0.001). Among pts with elevated FeCal ( >250mg/kg), median change from BL in FeCal concentration (mg/kg) at Wk12 were -800 and -86mg/kg for the GUS and PBO cohorts, respectively (p< 0.001); the proportions of pts achieving 50% and 75% reduction in FeCal levels (or ≤250 mg/kg) at Wk12 were higher for GUS-treated pts than those receiving PBO (51.1% vs 33.8% and 41.4% vs 23.6%; both p< 0.001). At Wk12, GUS-treated pts achieved CRP≤3mg/L and/or FeCal≤250mg/kg at higher rates than PBO-treated pts (Figure).
Discussion: Pts with moderately to severely active UC and elevated inflammatory markers treated with GUS 200mg IV induction showed greater improvement from BL in both CRP and FeCal levels compared with PBO. Differences were observed as early as the first assessments at Wk4 and continued through Wk12.

Figure: Figure. Proportions of patients achieving CRP ≤3 mg/L and FeCal ≤250 mg/kg through Week 12 among patients with elevated CRP (>3mg/L) or FeCal (>250mg/kg) at baseline
Disclosures:
Tadakazu Hisamatsu: AbbVie GK – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Alfresa Pharma Corporation and EA Pharma Co., Ltd – Grant/Research Support. Daiichi-Sankyo – Grant/Research Support. EA Pharma Co, Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Eli Lilly – Consultant. Gilead Sciences – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Janssen Pharmaceutical K.K. – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. JIMRO Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. KISSEI PHARMACEUTICAL CO., LTD – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Kyorin Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mitsubishi Tanabe Pharma Corporation – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Mochida Pharmacuetical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nichi-Iko Pharmaceutical Co., Ltd – honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Nippon Kayaku Co., Ltd – Grant/Research Support. Pfizer Japan Inc. – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Takeda Pharmaceutical Co., Ltd – Grant/Research Support, honoraria and had expenses paid to attend or give a presentation/advice at a meeting. Zeria Pharmaceutical Co., Ltd – Grant/Research Support.
Laurent Peyrin-Biroulet: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Allergan – personal fees. Alma – personal fees. Amgen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Applied Molecular Transport – personal fees. Arena – personal fees. Biogaran – Advisory Committee/Board Member, Consultant, Speakers Bureau. Biogen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Speakers Bureau. Bristol Myers Squibb – personal fees. Celgene – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. CTMA – Stock Options. Eli Lilly – Advisory Committee/Board Member, Consultant, Speakers Bureau. Enterome – personal fees. Enthera – personal fees. Ferring – Advisory Committee/Board Member, Consultant, Speakers Bureau. Forward Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Fresenius – personal fees. Genentech – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead – personal fees. H.A.C. Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Hikma – personal fees. Hospira/Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Index Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Lycera – Advisory Committee/Board Member, Consultant, Speakers Bureau. Merck – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mitsubishi – Advisory Committee/Board Member, Consultant, Speakers Bureau. MSD – Grant/Research Support, personal fees. Mylan – personal fees. Nestlé – personal fees. Norgine – Advisory Committee/Board Member, Consultant, Speakers Bureau. Oppilan Pharma – personal fees. OSE Immunotherapeutics – personal fees. Pfizer – personal fees. Pharmacosmos – personal fees. Roche – personal fees. Samsung Bioepis – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sandoz – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sterna – personal fees. Sublimity Therapeutics – personal fees. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Theravance – Advisory Committee/Board Member, Consultant, Speakers Bureau. Tillots – Advisory Committee/Board Member, Consultant, Speakers Bureau. Vifor – Advisory Committee/Board Member, Consultant, Speakers Bureau.
Axel Dignass: AbbVie – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Abivax – participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Amgen – Consultant. Arena Pharmaceuticals – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. Biogen – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant. Bristol Myers Squibb/Celgene – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees. CED Service GmbH – Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr Falk Foundation – Consultant, participation in clinical trials, review activities and manuscript preparation, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Fresenius Kabi – Consultant. Galapagos – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Gilead – participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. High5MD – Speakers Bureau. Janssen – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Lilly – Consultant. Materia Prima – Speakers Bureau. MedToday – Speakers Bureau. MSD – Consultant, Speakers Bureau. Pfizer – Consultant, participation in clinical trials, review activities such as data monitoring boards, statistical analysis and end point committees, Speakers Bureau. Pharmacosmos – Consultant. Roche/Genentech – Consultant. Sandoz/Hexal – Consultant. Streamed-Up – Speakers Bureau. Takeda – Consultant, manuscript preparation, Speakers Bureau. Thieme – manuscript preparation. Tillotts – Consultant, Speakers Bureau. UniMed Verlag – manuscript preparation. Vifor Pharma – Consultant, Speakers Bureau.
Kuan-Hsiang G. Huang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Matthew Germinaro: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Nicole Houck: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Ye Miao: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Hongyan Zhang: Johnson & Johnson – Employee, Stock Options, Stock-publicly held company(excluding mutual/index funds).
Marjorie Argollo: Abbvie – Consultant. Janssen – Consultant. Pfizer – Consultant. Sandoz – Consultant. Takeda – Consultant.
Ken Takeuchi: AbbVie – Grant/Research Support, Speakers Bureau. Celltrion – Speakers Bureau. EA pharma – Speakers Bureau. Janssen – Speakers Bureau. Kyorin – Speakers Bureau. Mochida – Speakers Bureau. Takeda – Grant/Research Support, Speakers Bureau. Tanabe Mitsubishi – Speakers Bureau. Thermo Fisher Diagnostics K.K. – Consultant. Zeria – Speakers Bureau.
Rafal Filip: Boxer – Consultant. Egis – research/education support. Janssen – lecture fees and research/education support. Takeda – lecture fees and research/education support. Zentiva – lecture fees.
Jessica Allegretti: Abbvie – Consultant, Speakers Bureau. Adiso Therapeutics – Consultant. Artizan – Consultant. Artugen Therapeutics – Consultant. Baccain – Consultant. Bristol-Myers Squibb/Celgene – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant. Finch Therapeutics – Consultant. Iterative Scopes – Consultant. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Merck – Consultant, Grant/Research Support. Morphic – Consultant. Pandion Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support. Roivant Sciences – Consultant. Seres Therapeutics – Consultant. Servatus – Consultant. Summit – Consultant.
Brian Feagan: AbbVie – Advisory Committee/Board Member, Consultant, Speakers Bureau. AbolerIS – Consultant. AgomAB Therapeutics – Consultant. Allianthera – Consultant. Amgen – Advisory Committee/Board Member, Consultant. AnaptysBio – Consultant. Applied Molecular Transport Inc. – Consultant. Arena Pharma – Consultant. Avir – Consultant. Azora Therapeutics – Consultant. Baxter – Consultant. BioJamp – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boston Pharma – Consultant. Bristol Myers Squibb – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Connect BioPharma – Consultant. Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1 – Advisory Committee/Board Member, Consultant. Eli Lilly – Consultant. Equillium – Consultant. Ermium – Consultant. Everest Clinical Research Corp. – Consultant. Ferring Pharmaceuticals – Consultant. First Wave – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Glenmark – Consultant. Gossamer Pharma – Consultant, stock shareholder. Hoffmann-LaRoche – Consultant. Hot Spot Therapeutics – Consultant. Imhotex – Consultant. ImmunExt – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Advisory Committee/Board Member, Consultant. Intact Therapeutics – Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. L.E.K. Consulting – Consultant. Landos Biopharma – Consultant. Leadiant – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Millennium – Consultant. MiroBio – Consultant. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. Novartis – Advisory Committee/Board Member. OM Pharma – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Otsuka – Consultant. Pandion Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Play to Know AG – Consultant. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Therapeutics and Diagnostics – Advisory Committee/Board Member, Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. RedHill Biopharma – Consultant. Redx – Consultant. Roche – Consultant. Sandoz – Consultant. Sanofi – Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Surrozen Inc. – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Speakers Bureau. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Theravance – Consultant. Tigenix – Consultant. Tillotts Pharma – Advisory Committee/Board Member, Consultant. UCB Pharma – Consultant. VHSquared Ltd. – Consultant. Viatris – Consultant. Western University, Alimentiv Inc – Employee. Ysios – Consultant. Ysopia – Consultant. Zealand Pharma – Consultant.
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, Speaker’s fees. Adiso Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. Arena pharmaceuticals – Consultant. Artizan Biosciences – Consultant. Artugen Therapeutics – Consultant. AstraZeneca – Consultant. Bacainn Therapeutics – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees and other support. Calibr – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Connect Biopharm – Consultant. Cytoki Pharma – Consultant. Eli Lilly – Consultant, speaking fees and other support. Enthera – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Galapagos – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. HMP Acquisition – Consultant. Imhotex – Consultant. Immunic – Consultant. InDex Pharmaceuticals – Consultant. Innovation Therapeutics – Consultant. Inotrem – Consultant. Ironwood Pharmaceuticals – Consultant. Janssen – Grant/Research Support, consulting and speaking fees and other support. Johnson & Johnson – Consultant. Kaleido Biosciences – Consultant. Kallyope – Consultant. Merck – Consultant. MiroBio – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. OSE Immunotherapeutics – Consultant. Pfizer Inc – Consultant, Grant/Research Support, speaking fees and other support. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. RedHill Biopharma – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Synlogic Operating Company – Consultant. Takeda – Grant/Research Support, consulting and speaking fees and other support. Target RWE – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. USWM Enterprises – Consultant. Ventyx Biosciences – Consultant, personal fees and stock options for consulting. Viela Bio – Consultant.
Tadakazu Hisamatsu, MD, PhD1, Laurent Peyrin-Biroulet, MD, PhD2, Axel Dignass, MD, PhD3, Kuan-Hsiang G. Huang, MD, PhD4, Matthew Germinaro, MD4, Nicole Houck, BS4, Ye Miao, MS4, Hongyan Zhang, PhD4, Marjorie Argollo, MD5, Ken Takeuchi, MD, PhD6, Rafal Filip, MD, PhD7, Jessica R.. Allegretti, MD, MPH8, Brian G. Feagan, MD9, Bruce E. Sands, MD, MS, FACG10. P2202 - Improvement in Inflammatory Biomarker Levels Through Week 12 in Moderately to Severely Active Ulcerative Colitis Patients Treated With Guselkumab: Results From the Phase 3 QUASAR Induction Study, ACG 2023 Annual Scientific Meeting Abstracts. Vancouver, BC, Canada: American College of Gastroenterology.
